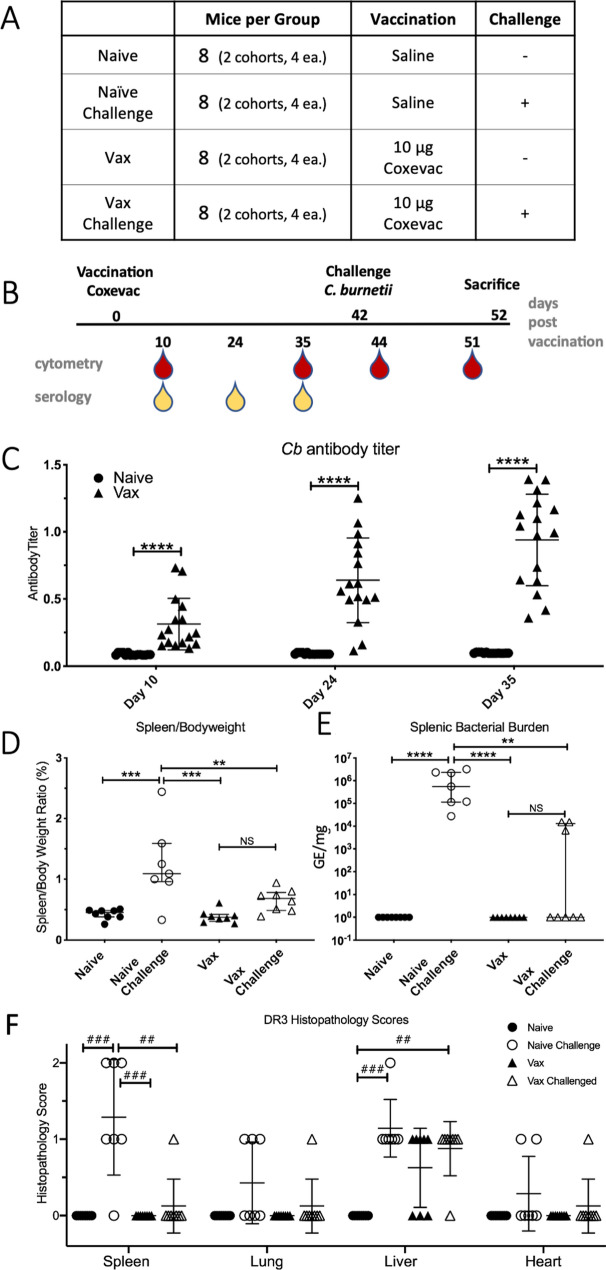
Figure 1.
Clinical outcomes of Coxevac vaccination and Cb challenge in tgHLA-DR3 mice. (A) Treatment groups and numbers of mice for the tgHLA-DR3 study (B) Experimental schedule. Mice were injected subcutaneously with saline or 10 µg Coxevac on day 0. After 42 days mice were challenged intranasally with live Cb. Whole blood was collected for CyTOF analysis on Day 10, 35, 44 and 51. Sera were collected for serology on Day 10, 24 and 35. (C) Antibody production against Cb was evaluated at Day 10, 24, and 35 post-vaccination by ELISA (D) Spleen-to-body-weight ratio and (E) spleen bacterial burden (genome equivalents (GE) determined by qPCR) were assessed for each of the experimental groups. Significant differences between experimental groups in panels (C–E) were assessed by one-way ANOVA with the Tukey post-hoc multiple comparison correction (****p = 0.0001, ***p < 0.0003, *p < 0.01). (F) Histopathological scores from lung, liver, spleen, and heart. Kruskal–Wallis test was used to compare between groups (###p = 0.0003, ##p = 0.005).