Abstract
Nerve hydrodissection (HD), a technique used when treating nerve entrapments, involves the injection of an anesthetic, saline, or 5% dextrose in water to separate the nerve from the surrounding tissue, fascia, or adjacent structures. Animal models suggest the potential for minimal compression to initiate and perpetuate neuropathic pain. Mechanical benefits of HD may relate to release of nervi nervorum or vasa nervorum compression. Pathologic nerves can be identified by examination or ultrasound visualization. The in-plane technique is the predominant and safest method for nerve HD. Five percent dextrose may be favored as the preferred injectate based on preliminary comparative-injectate literature, but additional research is critical. Literature-based hypotheses for a direct ameliorative effect of dextrose HD on neuropathic pain are presented.
Keywords: nerve hydrodissection, pain management, ultrasonography, neuropathic pain
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
The technique of high-resolution ultrasound (US)-guided hydrodissection (HD) of peripheral nerves has recently drawn the attention of the medical profession, especially in the fields of pain and musculoskeletal medicine. Randomized controlled trials published in high impact journals have suggested that this technique can safely and effectively treat carpal tunnel syndrome,1–4 the most extensively studied clinical condition treated by ultrasound-guided HD of peripheral nerves.1–8 Other clinical studies have also used this technique to treat neuropathic pain related to deep nervous structures or the neuraxial spine.9 In this article, we will review the animal model for nerve compression, injuries, or other conditions predisposing to compression-related symptoms, historical characteristics of neuropathic pain, and theoretical benefits of decompression. How to identify pathologic nerves, methods of ultrasound-guided decompression, and available literature related to the choice of an injectate and efficacy of HD will be summarized. Due to empirical and clinical trial evidence of a direct ameliorative effect of dextrose separate from decompression, hypotheses for such a direct effect will be discussed.
Nerve Compression Effects: Animal Model, Predisposing Conditions, and Theoretical Benefits of Decompression
Animal Model: Bennett’s Neuropathic Pain Model Demonstrates Peripheral Nerve Vulnerability to Mild Constrictive Effects
Nerve HD is a technique that uses high-resolution US-guided fluid injection to separate nerves from a surrounding or adjacent structure, usually the fascia, which is believed to constrict or irritate the nerve either during movement or at rest.10,11 The vulnerability of mixed sensory/motor nerves to circumferential compression was demonstrated by Bennett et al,12 who developed the most commonly studied animal model of neuropathic pain by placing self-dissolving ligatures about the sciatic nerve of rats. A key aspect of his approach was the use of no more than light constriction to avoid any visible restriction of epineural blood flow or significant indentation of the nerve surface; ensured by placement of ligatures that could be repositioned on the nerve with minimal effort.13 This light constriction led to prominent and rapid morphological changes, and development of allodynia and hyperesthesia, and provided a rationale to suspect that, in humans, peripheral nerves may be more vulnerable to light compression and entrapment effects at multiple locations than previously suspected or reported. Until a precise way to measure the pressure exerted on nerves by various types of compressive forces, including fascial compression, can be identified, Bennett’s consistently reproducible light-constriction animal model is the best available explanation how mild compressive effects in humans can result in neuropathic pain development, and why their release may result in therapeutic benefit.
Predisposing Conditions Which May Render the Peripheral Sensory Nerves Susceptible to Compressive Effects
Sports Injuries
- Sudden nerve elongation during sprain or strain injuries, which exceeds the stretch limit of the semi-elastic components of a nerve.
- Forced nerve movement through areas of fascial constrictions.
- Forced nerve movement around bony prominences; e.g., sudden movement of the common fibular nerve about the fibular head during an inversion sprain injury.
Osteophytosis/Tendinosis/Other Degenerative Changes
- Osteophytic changes which alter the course of a nerve, serving as a point of friction.
- Reduced flexibility in areas of degenerated soft tissue may alter free movement of nerves coursing through that area.
- Sensitization of nerves within areas of chronic tendinosis or ligamentosis.
Post-Fracture or Post-Surgical Pain
- Central or peripheral sensitization due to uncontrolled pain.
- Stretch injury to nerves occurring in the process of required nerve retraction during open surgery.
- Direct nerve contusions at the time of injury with secondary nerve swelling resulting in abnormal friction/compression during nerve movement.
- Altered gait post injury resulting in nerve irritation through overuse or misuse of extremities.
- Scar or fibrosis about surgical/fracture sites, altering the normal nerve course.
Other
The patient’s history may not reveal a predisposing cause or predisposition to nerve compression. Empirically this appears to be common but epidemiologic studies are lacking.
Characteristics of Neuropathic Pain and Theoretical Benefits of Decompression
Definition and Characteristics of Neuropathic Pain
Neuropathic pain is defined as pain caused by a lesion or disease of the somatosensory system.14,15 It usually has the following characteristics:
Deep-seated pain with poor localization of the source of pain.
The degree of pain is usually not proportional to the degree of tissue/nerve damage.
The pain can be described as soreness, numbness, or an electric shock sensation, in the presence of hyperalgesia and/or allodynia.
A sensation of cold or heat can be felt in the affected region, and the skin in the affected area may appear bluish, similar in appearance to venous stasis.
Potential Benefits of Decompression on Nervi Nervorum, Vasa Nervorum, or Lymphatic Drainage
A potential benefit of the use of fluid (hydro) to separate nerves from the surrounding soft tissue (dissection) to treat neuropathic pain is the release of pressure on the “free nerves supplying the main nerves,” which are called “nervi nervorum,”16–20 which are located outside the epineurium. The nervi nervorum innervate and regulate the function and discharge of sensory, motor, or mixed-modality nerves (Figure 1). “Vasa nervorum,” are small blood vessels, also located outside the epineurium. The arteries supply nutrients to the peripheral nerve and the veins drain away the metabolites from these nerves.21–23 Mild compression of the vasa nervorum would first affect venous outflow, with potential stasis and accumulation of toxins at the affected part of the nerve. Lymphatic drainage, which may be present outside the epineurium (Figure 1), would also be subject to compressive effects. Therefore, the primary objective of HD is to release the entrapment of the peripheral nerves by hydrodissecting the nerves.10
Figure 1.
Illustration of the “nervi nervorum” and “vasa nervorum” outside the epineurium.
Identification of Pathologic Nerves and HD Methods
Identification of Pathologic Nerves
The pathologic nerves/entrapped nerves are usually swollen compared to their opposite extremity counterparts in those with unilateral symptoms. The cross-sectional areas (CSA) of the entrapped nerves may be double or even triple in size in comparison to healthy nerves. Examples of this are shown in Figure 2(left)–4.24,25
One of the fascicles of the entrapped/diseased nerve is swollen and much bigger than the rest of the fascicles within the same nerve (Figure 2(right), 5, and 6).24,25 Video 1 shows US tracking from distal to proximal of a common fibular (peroneal) nerve (CPN) with a swollen fascicle.
Direct digital palpation of the suspected swollen nerve or suspected nerve with swollen fascicles reproduces the neuropathic pain experienced by the patient.
Using continuous dynamic US, a snapping/sudden motion of a nerve against a surrounding bone, ligament, or tendon is observed, reproducing the neuropathic pain experienced by the patient. Video 2 shows direct digital palpation and snapping of the common fibular (peroneal) nerve (CPN) against the fabella when the knee is flexed and extended. Dynamic US of the nerves shows the loss of relative movement of nerves in relation to their surrounding structures. A common example of this is the loss of the “seesaw sign” of the tibial nerve and common fibular nerves in the sciatic nerve sleeve during ankle-planter flexion and dorsiflexion.26 Video 3 shows dynamic US visualization of the loss of the “seesaw sign” during ankle plantar/dorsiflexion.
If the patient has no phobia of needles, US-guided dry needling can be utilized. The needle is placed as close as possible to the diseased part of the nerve and stimulated by vibration or electricity while observing for reproducibility of the neuropathic pain experienced by the patient. Video 4 shows the dry needle procedure of a pathologic nerve with stimulation under US guidance.
Figure 2.
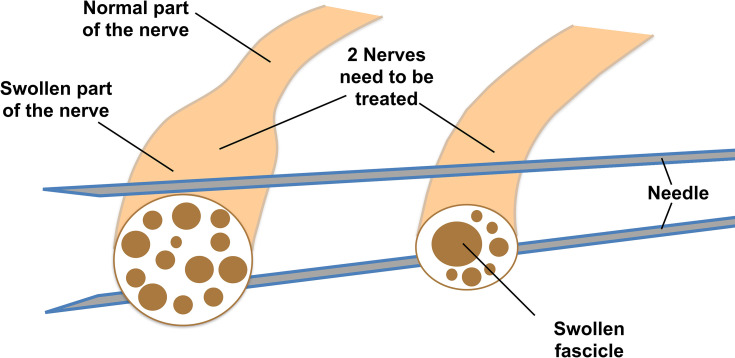
Illustration of two examples of abnormal nerves. The nerve on the left side has part of the nerve with the whole cross-sectional area (CSA) double, or even more, than normal, but the fascicles of the nerve are relatively normal. The nerve on the right side shows the CSA of the nerve is normal or slightly enlarged but one or more of the fascicles inside the nerve is much larger. The needles in this illustration will be referred to later to illustrate how method 1 can be used to hydrodissect two parallel nerves (such as tibial and fibular nerves at popliteal fossae).
Figure 4.
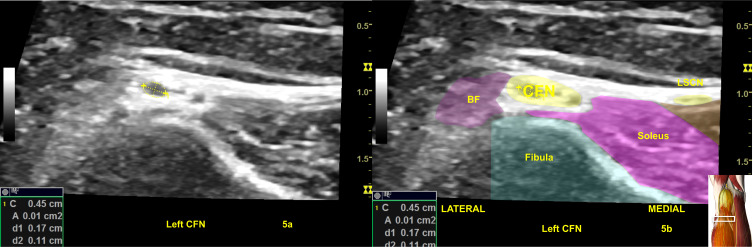
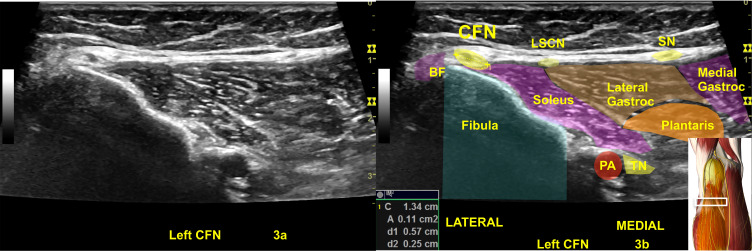
An abnormal right common fibular nerve (CFN) with twice the normal CSA (22 mm2) at the fibular head (A, B) of the same patient in Figure 3.24,25 4a is the original ultrasound image. The (B) shows the CSA of the abnormal right CFN and the color shadings with labels for sonoanatomy, Image is courtesy of 3D4Medical’s Essential Anatomy 5 app.
Abbreviations: BF, biceps femoris; Gastroc, gastrocnemius; LSCN, lateral sural cutaneous nerve; PA, popliteal artery; SN, sural nerve; TN, tibial nerve.
Figure 5.
(A) Shows a normal common fibular nerve (CFN) at fibular head of Figure 3 with normal fascicles, the most prominent fascicle at the upper limit of normal (1 mm2) in cross-sectional area. The color shading in (B) with labeling are for illustration purpose. Image is courtesy of 3D4Medical’s Essential Anatomy 5 app
Abbreviations: BF, biceps femoris; Gastroc, gastrocnemius; LSCN, lateral sural cutaneous nerve.
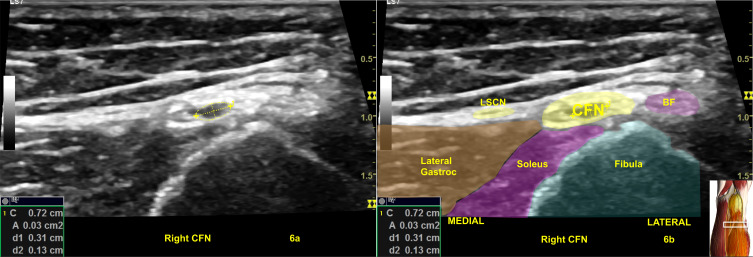
Figure 6.
(A) Illustrates a swollen common fibular nerve (CFN) at fibular head of Figure 4 with a swollen nerve fascicle with a cross-sectional area of 3 mm2. The color shading in (B) with labeling are for illustration purpose. Image is courtesy of 3D4Medical’s Essential Anatomy 5 app
Abbreviations: BF, biceps femoris; Gastroc, gastrocnemius; LSCN, lateral sural cutaneous nerve.
HD Methods
There are two primary methods of US-guided HD of peripheral nerves.27 Literature review revealed no in-depth comparison of the performance of these techniques with respect to the learning curve, effectiveness, and safety of each.
Method 1 (in-Plane Approach, Needle Perpendicular to the Long Axis of the Nerve)
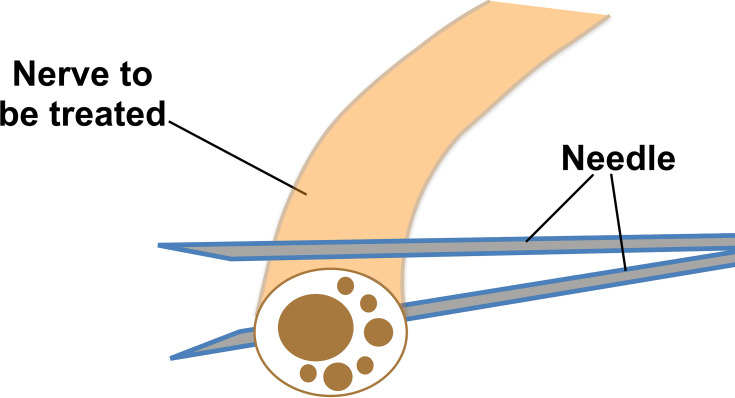
Generally, when using method 1 for HD of nerves, the needle and probe are both perpendicular to the long axis of the nerve. The needle is in-plane to the transducer, and the tissues above and below the nerves are hydrodissected. The needle first approaches the inferior surface of the nerve with the needle bevel positioned up, and the pressure of the injectate is used to open the soft tissues around the nerve layer by layer until the injectate surrounds the epineurium. Since flexible hypodermic needles with bevels are typically used, it is safer to approach the inferior surface of the nerve bevel up as the resistance of the soft tissue will generally force the needle to go deep, and avoid damaging the inferior part of the nerve.28 The same process is repeated with the needle approaching from the superior surface of the nerve, with the needle bevel positioned down (Figure 7). The hypodermic needle is bevel down so that the resistance of the soft tissue will force the needle to move more superficial and avoid injuring the superior part of the nerve.28 The hydrodissected nerve appears oval and surrounded by anechoic fluid on US when the release is completed. Video 5 shows the practice of this technique using method 1 (in-plane approach, needle perpendicular to the long axis of the nerve) in clinical situations. A 25-gauge 50 mm, or 22-gauge 70–100 needle is used, depending on the depth of the nerve, and keeping in mind the benefit from an enhanced needle echogenicity that results from reduction of the angle between the probe and the path of needle movement.
Figure 7.
Needle position for method 1 of hydrodissection (HD) of nerves. With the “in-plane” technique, first, the inferior surface of the nerve is hydrodissected with the needle bevel positioned up; and thereafter, the superior surface of the nerve is hydrodissected with the needle bevel positioned down.
Clinical Pearls Related to HD in General and Method 1 Specifically
The basic principle of US-guided HD of peripheral nerves is that the fluid injectate, not the needle, is the tool used to separate the soft tissues. Therefore, after administering local anesthetics at the superficial entry point of the needle, it is essential to advance the needle slowly and visualize the needle at all times during the procedure, particularly during needle advancement, during which the physician will continually inject fluid. The injectate incrementally separates the soft tissues in front of the needle, followed by movement of the needle tip into the resultant fluid space.
Slow needle advancement while injecting also minimizes disruption of important soft tissues; e.g., vascular bundles and other nerves, running parallel to the target nerve to be hydrodissected. It also maximizes comfort through allowing more time for surrounding tissues to be gently pushed aside by a continuous fluid jet. This facilitates performance of hydrodissection without a lidocaine component.9
In addition to observing the bevel position, continuous needle tip observation, continuous injection before and during needle movement, and slow needle advancement, safety is further enhanced by encouraging a verbal commentary from the patient during which they describe what sensations they experience during the procedure. It is best to advise patients that, when the nerve is hydrodissected from the surrounding soft tissues, they will commonly experience aching, numbness, a burning sensation, or cramping in the distribution of the nerve, particularly at the moment of fascial release, and rarely an electrical sensation if the epineurium is lightly contacted or when scar tissue is peeled off the epineurium.
It is the author’s experience that method 1 requires less injectate volume to separate the nerve from the surrounding soft tissues. If the nerve is tethered significantly to the soft tissue; e.g., scar tissue, method 1 can be used to safely detach the nerve from the scar tissue. Method 1 can be learned rapidly, proportional to the amount of hands-on practice of needling technique, supplemented as feasible by cadaver workshops.
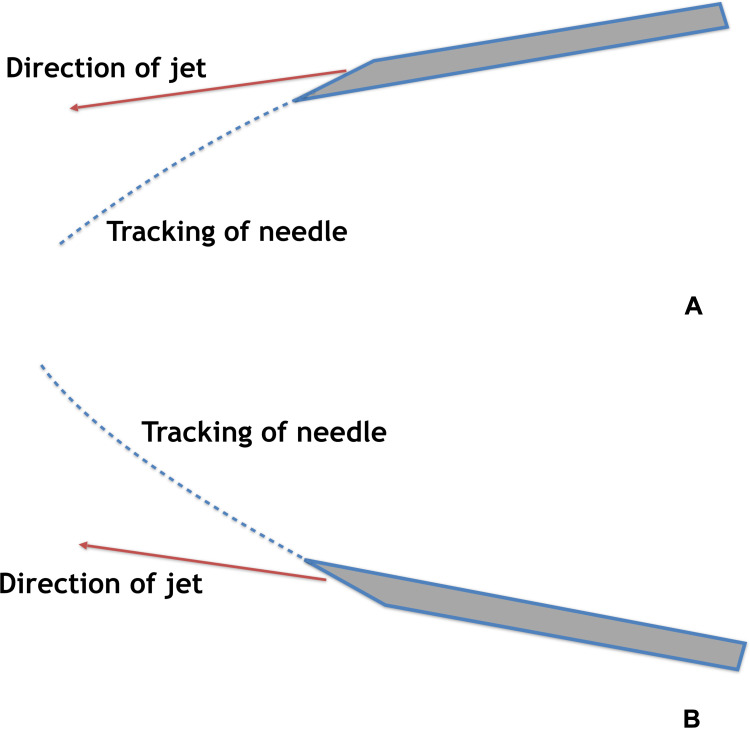
Bevel position may impact needle direction/tracking, especially if a long and thin needle is used (Figure 8).28 If the bevel of a needle is positioned on the side, it is the authors’ experience that the needle will tend to bend to the opposite side, out of alignment with the target, and out of the narrow viewing plane of the ultrasound probe.
Usually, HD of the nerve is initiated from the site where the nerve is most severely damaged or trapped. If the diseased nerve or the damaged/entrapped part of the nerve is long, the same entry point can be used, with the transducer and needle pivoted to the proximal portion of the nerve, and after that, to the distal part of the nerve to repeat the HD process (Figure 9).
Method 1 can be used for HD of 2 to 3 nerves running parallel to each other if all nerves require treatment simultaneously (Figure 2). Video 6 shows the use of method 1 to treat 2 or 3 diseased nerves running parallel to each other.
Figure 8.
Effect of bevel position on the direction of tracking of the needle. (A) Effects of bevel position up and (B) effects of bevel position down.
Figure 9.
Sequence of hydrodissection (HD) of the diseased nerve using method 1. First, HD is initiated from the site where the nerve is most severely damaged or trapped, thereafter, using the same needle entry point, pivot the probe and the needle to the more proximal and/or distal part of the nerve and repeat the HD.
Method 2 (Out-of-Plane with Subsequent in-Plane Approach)
During the performance of method 2, the needle is parallel to the long axis of the nerve, and the probe is first perpendicular and then parallel to the long axis of the nerve. An “out-of-plane” technique is used to HD the nerve from the surrounding tissues, confirming that the nerve is freed from the surrounding soft tissues by the visualization of anechoic fluid surrounding the nerve (both above and below the nerve). Subsequently, the probe is turned “in-plane” toward the nerve, the needle tip is guided back to the top of the nerve, and fluid is injected above it, with the bevel positioned down when approaching the nerve to avoid making accidental contact with the nerve. The injected fluid should be visualized to be tracking above and below the nerve. An illustration of the directions of the needle during method 2 is shown in Figure 10. Video 7 shows an example of one of the practice of method 2 for HD of nerves. Video 8 shows example two of the practice of method 2 for HD of nerves.
Figure 10.
Relative direction and movement of the needle when using method 2 for hydrodissection (HD) of nerves. This shows the initial out-of-plane portion of method 2 with HD of the nerve on either side until injectate is seen surrounding the nerve, at which point the probe position is changed to in-plane with the nerve to HD the space above the nerve.
Clinical Pearls Related to Method 2
The end goal of HD of nerves is confirmed by visualization of anechoic injectate above, below, proximal, and distal to the hydrodissected nerves and an alteration in nerve shape. Either method can accomplish this, but method 2 can separate a comparatively longer length of nerve from surrounding soft tissue through a single insertion point.
Method 2 for HD of nerves requires good “out-of-plane” and “in-plane” techniques, and often the ability to switch back and forth between the two. If the needle tip is not visualized, and the doctor keeps advancing the needle, impalement of the nerve may occur. The learning process for method 2 is usually much longer than method 1. Extensive practice of this technique in cadaver courses and a high comfort level with out-of-plane visualization is recommended prior to attempting method 2. If not proficient in this technique, a doctor with good method 1 skill can perform in-plane HD perpendicular to the short axis of the nerve first, followed by HD of the nerve in-plane with the needle and transducer parallel to the long axis of the nerve through another needle entry point. Another alternative, using an in-plane technique through the same needle entry point, is to utilize several needle redirections as described under pearls for method 1. A third option is to simply use method 1 with different entry points to HD the nerve at several locations along the area of constriction.
Safety of US-Guided HD of Nerves
Whenever a doctor is injecting around the nerve, nerve injury is a potential complication.29 Jeng et al suggest that nerve damage is rare due to the polyfascicular architecture of the peripheral nerve and nerve fiber dispersal within the nerve.29 The importance of avoiding nerve contact cannot be overemphasized, and the advantages of hydrodissection without lidocaine may be considerable, in that it allows the clinician to hydrodissect continually and liberally ahead of the needle, without concern for anesthetic toxicity. Nevertheless, HD of nerves is not a technique for beginners in US-guided pain interventions, requiring relatively advanced skills in US-guided needling techniques. Cadaveric injection experience is crucial, and the authors stress repetitively that the injecting pressure should be the separating agent to release the soft tissues tethered to the nerves, not the needle itself.
Current Literature
HD with Normal Saline (NS) in Carpal Tunnel Syndrome (CTS) Outperformed a NS Control Injection
Traditionally, a large volume of NS and a small volume of steroid and local anesthetic solution are used for HD of nerves.5,6,30 HD alone appears to be beneficial, as shown in a clinical trial by Wu et al in patients with mild-to-moderate carpal tunnel syndrome (CTS). They compared the effect of HD with 5 mL NS to subcutaneous injection of 5 mL NS above the carpal tunnel.3 The Boston Carpal Tunnel Syndrome Questionnaire (BCTSQ) mean subscores for symptom severity and function were followed, with a range from 1 for no symptoms to 5 for the most severe symptoms for each subscale. HD with NS outperformed subcutaneous saline injection at 6 months for improvement in symptom severity (−0.6 ± 0.2 vs. −0.2 ± 0.1; p = 0.024), functional status (−0.6 ± 0.1 vs. −0.2 ± 0.1; p = 0.041), and edema as determined by significantly more reduction in cross-sectional area (CSA) of the median nerve in the intracarpal injection group (−1.3 ± 0.03 vs. 0.3 ± 0.1 mm3; p < 0.001).
HD with Dextrose 5% in Water (D5W) in CTS Outperformed HD with NS or Triamcinolone
Wu et al also compared a single median nerve HD with 5 mL of D5W to HD with 5 mL of NS, and used the total BCTSQ subscore range (11–55 for symptoms and 8 to 40 for function) instead of the mean BCTSQ1 as their outcome measure. Dextrose HD outperformed NS HD at six months for improvement in symptom severity (−14.9 ± 1.2 vs. 6.5 ± 1.5; p < 0.001) and functional status (−10.4 ± 0.8 vs. −2.9 ± 0.09; p < 0.001). The CSA improved (decreased) significantly more in the intracarpal dextrose group (−2.2 ± 0.3 vs. −1.2 ± −0.2 mm3; p < 0.004).
Wu et al next compared a single median nerve HD with 5 mL of D5W to 5 mL HD with 5 mL containing 3 mL of 10mg/mL triamcinolone plus 2 mL NS,2 and dextrose outperformed triamcinolone at six months for mean difference in total BCTSQ symptom (−13.5 vs. 3.9; p <0.005) and function subscores (−9.4 vs. −3.0; p = <0.001) (standard deviations of change scores were not listed.) The cross-sectional area improved in both groups significantly with no between-group difference (−2.1 vs. – 1.6 mm3; p = 0.30).
HD with Hyaluronidase in CTS Outperformed HD with NS
Elawamy et al compared a single median nerve HD with 1500 IU of a proprietary hyaluronidase plus 10 mL NS to HD with 10 mL NS.7 At 6 months the hyaluronidase group outperformed NS alone for mean difference in total BCTSQ symptom (−13.9 vs −0.3; p <0.001) and function subscores (−10.1 vs.+1.4; p <0.001) and the CSA area improved (decreased) only in the hyaluronidase group (−2.7 vs. −0.1 mm3; p < 0.05).
HD with PRP in CTS Outperformed Splint Only Use but Not HD with D5W
Wu et al compared a single median nerve HD with 3 mL of PRP to 8 hours of night splint use daily in randomized open-label fashion. PRP outperformed splint use at six months for mean difference in total BCTSQ symptom (−11.8 ±1.2 vs. 8.7 ± 0.9; p =0.045) and function (−8.7 ± 0.9 vs. −5.2 ± 0.5; p = 0.001) subscores. The cross-sectional area improved in both groups significantly, with no between-group difference (−3.1 ± 0.2 vs. – 2.0 ± 0.3 mm3; p = 0.004).4 Shen et al compared a single median nerve HD with 3 mL of PRP to HD with 3 mL D5W.8 Using the mean subscores for the BCTSQ, both PRP and D5W HD resulted in noteworthy and statistically similar improvements at 6 months in symptom severity (−1.2 ± 0.2 vs. −1.0 ± 0.1; p = 0.447) and functional status (−1.2 ± 0.1 vs. −1.1 ± 0.1; p = 0.267), although edema was significantly more in the PRP injection group (−3.3 ± 0.03 vs. −1.9 ± 0.4 mm3; p =018).8 Raeissadat et al compared single injection PRP with splint versus splint use without injection. However, HD was not utilized during injection, only 1 mL PRP was injected, and follow-up was only 10 weeks.31 Senna et al compared a single median nerve HD with 2 mL PRP to 1 mL of methylprednisolone with ultrasound guidance, with no description of HD and follow-up of only 3 months.32 Catapano et al, in a recent metaanalysis, commented favorably on the potential for benefit of PRP in the treatment of CTS.33
Non-CTS-Research Observations and Summary of Research Status
Other studies have supported the use and efficacy of dextrose solution to treat neuropathic pain. Injection of 10 mL of D5W into the caudal epidural space versus 10 mL of normal saline has been demonstrated by Smigel et al to result in prompt and significantly more pain improvement as measured on a 0–10 numerical rating scale from 15 minutes post-injection (4.4 ± 1.7 vs. 2.4 ± 2.8 points; p = 0.015) through 48 hours post-injection (3.0 ± 2.3 vs.1.0 ± 2.1 points; p = 0.012).34 In a subsequent open-label study, the pattern and degree of pain relief with dextrose was similar after each injection, with a cumulative pain improvement of 3.4 ± 2.3 points (52%) on the 0–10 NRS scale for pain, and functional improvement 18.2 ± 16.4% (42%) on the 0–100 Oswestry Disability Index at 12 months.35
The typical injectate volume for research on HD of the median nerve in the carpal tunnel is 10 mL or less.1–4,7,8 However, according to the authors’ experience, a much larger volume of injectate (typically 20–30 mL) needs to be used to completely release the nerve from the surrounding soft tissues to achieve an oval appearance, as most nerves and plexi are not located in such a confined area as the carpal tunnel. Our clinical experience was summarized in a retrospective usual-care quality-assurance study data collection.9 Twenty-six consecutive patients with severe (8.3 ± 1.3 on a 0–10 NRS scale), chronic (mean 16±12.2 months) neuropathic pain were treated using hydrodissection with D5W of indicated nerve roots and plexi, without lidocaine inclusion. At 2-month follow-up after their last treatment, patients reported an improvement in pain of 6.4 ± 1.7 points (8.3± 1.3 before treatment to 1.9 ± 1.7 points after treatment), for a pain percentage improvement of 77%. The mean number of treatments required for a satisfactory response (3.8 ± 2.6 treatments) and mean treatment duration to 2-month follow-up of 9.7 ± 7.8 months are consistent with our current clinical experience. Two additional observations are of particular interest. One was that, in the absence of lidocaine, multiple procedures were able to be performed simultaneously, if deemed necessary for a more complete approach to nociceptive sources. A second observation was that marked analgesia resulted after each treatment within 15 minutes (88.1% ± 9.8%), consistent with the analgesic effect of dextrose reported by Smigel et al in their randomized caudal epidural study.34 Given the benefit of injection at the nerve root and plexi level, a mechanism of action of 5% dextrose injection at the somatosensory system at the dorsal root level has been proposed.34
Conclusions from the randomized controlled clinical trials in CTS are limited due to small study sizes and a 6-month duration of follow-up. In addition, other clinicians that perform hydrodissection routinely may not be convinced that a single injection of the volume of injection listed in the clinical HD trials will be adequate to expect a consistent clinical and electromyographic benefit in future corroborative studies. Another limitation of all clinical trials of HD to this point is that all of the injectates evaluated other than D5W have a volume limitation; e.g. steroid congeners, hyaluronidase or PRP, making them unsuitable for multiple large volume/multiple procedure applications. For that reason, and potentially cost efficacy reasons, D5W may be the primary injectate, followed by addition of second injectate after initial HD has been performed. Further research on current primary injectates is of critical importance.
Hypotheses for a Direct Ameliorative Effect of Dextrose on Neuropathic Pain
Several hypotheses have been proposed to explain the effect of dextrose solution on treating neuropathic pain such as:
Downregulation of the Transient Receptor Potential Vanilloid Receptor-1 (TRPV1) Ion Channel or Reduction of Its Downstream Mechanism of Action
Upregulation (persistent opening) of the TRPV1 ion channel is strongly associated with the persistence of chronic neuropathic pain.36 The TRPV1 ion channel was previously called the capsaicin receptor because no other ion channels are affected by capsaicin.37 Capsaicin causes a characteristic burning sensation by upregulating the TRPV1 channel. Mannitol, a 6-carbon-atom sugar, has been found to reduce the burning sensation after exposure to capsaicin, suggesting an antagonistic (calming) effect on TRPV1 upregulation, either directly or by downstream effect.38 Dextrose, similar in structure to mannitol, has empirically been observed to have a similar effect, although it has not been formally tested using the capsaicin model developed by Bertrand et al.38
Correction of Perineural/Intraneural Glycopenia
Chronic neuropathic pain may signify glycopenia around the corresponding nerve(s). Injecting dextrose may promptly correct this glycopenia and consequently reduce neuropathic pain. Moreover, 40% of our peripheral somatosensory nervous system is comprised of small capsaicin-sensitive nerves (nerves with the TRPV1 ion channels on their surface), which are predominantly C fibers, and have an apparent homeostatic role in monitoring the level of systemic dextrose.39 Both the brain and peripheral nerves have a high and constant requirement for glucose.11 MacIver reported that when isolated C fibers are exposed in vitro to a hypoglycemic environment by substituting D-glucose with non-metabolizable L-glucose, they demonstrate a dramatic (653±23%) increase in discharge frequency within 5 minutes, maximized after 15 minutes. The C fiber firing rate returned to baseline within 2 minutes of replacement of D-glucose in the culture solution.40 MacIver explains these prompt changes in neural firing rates by reminding us of the central role of D-glucose metabolism in provision of ATP to power the cellular Na+ -K+ pump in animal and human cells. Hypoglycemia results in reduced activity of the ATP dependent Na+-K+ pump, resulting in a progressive nerve depolarization and hyperexcitability.
Potential Improvement of Nerve Mobility Through US Hydrodissection
A cadaveric study done in Mayo clinic showed that HD can decrease the gliding resistance of the median nerve within the carpal tunnel, supporting the concept that HD may result in a beneficial mechanical change in nerve movement.41 However, the gliding resistance was measured immediately after hydrodissection and does not offer proof of a sustainable benefit. At this time that can be implied only indirectly by sustainable symptomatic benefit, and improvement of neural edema and nerve conduction parameters.
Conclusion
Bennett’s animal model of neuropathic pain is the most well-known and utilized animal model. Neuropathic pain results from such minimal compression that it supports the concept that minimal nerve compression is capable of creating structural changes in nerves as well as neuropathic pain. Many conditions can increase the susceptibility of sensory nerves to compression. A direct mechanical benefit from nerve release may result from the restoration of nervi or vasa nervorum function though the release of pressure effects. Examination and ultrasound visualization are jointly helpful to identify pathologic nerves. In-plane technique (method 1) is recommended as the primary/safer approach with key features of using the injectate jet to dissect the soft tissue in front of the needle, and fully releasing fascia until the nerve appearance is rounded and the nerve is completely surrounded by injectate fluid. The injectate of preference may be D5W for most applications, based on preliminary literature findings evaluating comparative injectates, empirical and clinical evidence of a direct analgesic effect of dextrose separate from a hydrodissection mechanism, and the ability of D5W to be used for high volume and multiple-nerve applications. The mechanism of benefit of HD for benefit in neurogenic pain has not been established and will require substantial basic science research.
Acknowledgments
The authors need to acknowledge Dr. Dick Hui for drawing Figure 1.
Figures 3–6 have been courtesy of 3D4Medical’s Essential Anatomy 5 app for illustrating the transducers positions.
Figure 3.
A normal left common fibular nerve (CFN) with a cross-sectional area (CSA) at the upper limits of normal (11 mm2) at fibular head (A and B).24,25 3a is the original ultrasound image, (B) Shows the highlighted CSA of the normal left CFN and the color shadings with labels for sonoanatomy, Image is courtesy of 3D4Medical’s Essential Anatomy 5 app.
Abbreviations: BF, biceps femoris; Gastroc, gastrocnemius; LSCN, lateral sural cutaneous nerve; PA, popliteal artery; SN, sural nerve; TN, tibial nerve.
Dr. Lam has the ownerships for all the figures and videos in this manuscript.
Funding Statement
This research received no external funding.
Disclosure
Some of the materials and pictures contained in this manuscript have been used in previous presentations during international academic conferences. The most recent one was the Annual Conference of the Australian Association of Musculoskeletal Medicine on 24–27 October 2019 in Brisbane, Australia. The previous one was the International Symposium of Ultrasound for Regional Anesthesia and Pain Medicine (ISURA 2019) on 9–11 May 2019 in Porto, Portugal. The authors report no conflicts of interest in this work.
References
- 1.Wu Y-T, Ho T-Y, Chou Y-C, et al. Six-month efficacy of perineural dextrose for carpal tunnel syndrome: a prospective, randomized, double-blind, controlled trial. Mayo Clin Proc. 2017;92(8):1179–1189. doi: 10.1016/j.mayocp.2017.05.025 [DOI] [PubMed] [Google Scholar]
- 2.Wu YT, Ke MJ, Ho TY, Li TY, Shen YP, Chen LC. Randomized double-blinded clinical trial of 5% dextrose versus triamcinolone injection for carpal tunnel syndrome patients. Ann Neurol. 2018;84(4):601–610. doi: 10.1002/ana.25332 [DOI] [PubMed] [Google Scholar]
- 3.Wu YT, Chen SR, Li TY, et al. Nerve hydrodissection for carpal tunnel syndrome: a prospective, randomized, double-blind, controlled trial. Muscle Nerve. 2019;59(2):174–180. doi: 10.1002/mus.26358 [DOI] [PubMed] [Google Scholar]
- 4.Wu Y-T, Ho T-Y, Chou Y-C, et al. Six-month efficacy of platelet-rich plasma for carpal tunnel syndrome: a prospective randomized, single-blind controlled trial. Sci Rep. 2017;7(1):94. doi: 10.1038/s41598-017-00224-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malone DGCTB, Wei N. Ultrasound-guided percutaneous injection, hydrodissection, and fenestration for carpal tunnel syndrome: description of a new technique. J Appl Res. 2010;10(3):107–114. [Google Scholar]
- 6.McShane JM, Slaff S, Gold JE, Nazarian LN. Sonographically guided percutaneous needle release of the carpal tunnel for treatment of carpal tunnel syndrome: preliminary report. J Ultrasound Med. 2012;31(9):1341–1349. doi: 10.7863/jum.2012.31.9.1341 [DOI] [PubMed] [Google Scholar]
- 7.Elawamy A, Hassanien M, Hamed A, et al. Efficacy of hyalase hydrodissection in the treatment of carpal tunnel syndrome: a randomized, double-blind, controlled, clinical trial. Pain Physician. 2020;23(2):E175–E183. [PubMed] [Google Scholar]
- 8.Shen YP, Li TY, Chou YC, et al. Comparison of perineural platelet-rich plasma and dextrose injections for moderate carpal tunnel syndrome: a prospective randomized, single-blind, head-to-head comparative trial. J Tissue Eng Regen Med. 2019;13(11):2009–2017. doi: 10.1002/term.2950 [DOI] [PubMed] [Google Scholar]
- 9.Lam SKH, Reeves KD, Cheng AL. Transition from deep regional blocks toward deep nerve hydrodissection in the upper body and torso: method description and results from a retrospective chart review of the analgesic effect of 5% dextrose water as the primary hydrodissection injectate to enhance safety. Biomed Res Int. 2017;2017:7920438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cass SP. Ultrasound-guided nerve hydrodissection: what is it? A review of the literature. Curr Sports Med Rep. 2016;15(1):20–22. doi: 10.1249/JSR.0000000000000226 [DOI] [PubMed] [Google Scholar]
- 11.Andreone BJ, Lacoste B, Gu C. Neuronal and vascular interactions. Annu Rev Neurosci. 2015;38(1):25–46. doi: 10.1146/annurev-neuro-071714-033835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi: 10.1016/0304-3959(88)90209-6 [DOI] [PubMed] [Google Scholar]
- 13.Bennett GJCJ, Honore M, Seltzer Z. Models of neuropathic pain in the rat. Curr Protoc Neurosci. 2003;22(1):9–14. [DOI] [PubMed] [Google Scholar]
- 14.Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599–1606. doi: 10.1097/j.pain.0000000000000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59 [DOI] [PubMed] [Google Scholar]
- 16.Bradshaw JM. Lecture on nerve-stretching for the relief or cure of pain. Br Med J. 1883;2(1198):1173–1179. doi: 10.1136/bmj.2.1198.1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugar OVH. Victor Horsley, John Marshall, nerve stretching, and the nervi nervorum. Surg Neurol. 1990;34(3):184–187. doi: 10.1016/0090-3019(90)90071-V [DOI] [PubMed] [Google Scholar]
- 18.Victor H. Preliminary communication on the existence of sensory nerves in nerve trunks. True “nervi nervorum”. Br Med J. 1884;1:166. [Google Scholar]
- 19.Carrero G, Moon JK. Fascicular anatomy, nervi nervorum, and paresthesia. Reg Anesth Pain Med. 2003;1:72–73. [PubMed] [Google Scholar]
- 20.Vilensky JAGS, Gilman S, Casey K. Sir Victor Horsley, Mr John Marshall, the nervi nervorum, and pain: more than a century ahead of their time. Arch Neurol. 2005;62(3):499–501. doi: 10.1001/archneur.62.3.499 [DOI] [PubMed] [Google Scholar]
- 21.Mizisin APWA, Weerasuriya A. Homeostatic regulation of the endoneurial microenvironment during development, aging and in response to trauma, disease and toxic insult. Acta Neuropathol. 2011;121(3):291–312. doi: 10.1007/s00401-010-0783-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yali Jia TKB, Wang RK. Label-free 3D optical microangiography imaging of functional vasa nervorum and peripheral microvascular tree in the hind limb of diabetic mice. J Innov Opt Health Sci. 2010;13(4):307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felten DLSMM. Peripheral Nervous System. Netter’s Atlas of Neuroscience (Third Edition). 2016:153–231. Elsevier. [Google Scholar]
- 24.Kim JY, Song S, Park HJ, Rhee WI, Won SJ. Diagnostic cutoff value for ultrasonography of the common fibular neuropathy at the fibular head. Ann Rehabil Med. 2016;40(6):1057–1063. doi: 10.5535/arm.2016.40.6.1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bedewi MA, Abodonya A, Kotb M, et al. Estimation of ultrasound reference values for the lower limb peripheral nerves in adults: a cross-sectional study. Medicine. 2018;97(12):e0179. doi: 10.1097/MD.0000000000010179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam SKH, Hung C-Y, Clark TB. Loss of the “Seesaw Sign” of the sciatic nerve in a marathon runner complaining of hamstring cramping. Pain Med. 2020;21(2):e247–e248. doi: 10.1093/pm/pnz283 [DOI] [PubMed] [Google Scholar]
- 27.Soneji N, Peng PW. Ultrasound-guided pain interventions – a review of techniques for peripheral nerves. Korean J Pain. 2013;26(2):111–124. doi: 10.3344/kjp.2013.26.2.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misra S, Reed KB, Schafer BW, Ramesh KT, Okamura AM. Mechanics of flexible needles robotically steered through soft tissue. Int J Rob Res. 2010;29(13):1640–1660. doi: 10.1177/0278364910369714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeng CL, Torrillo TM, Rosenblatt MA. Complications of peripheral nerve blocks. Br J Anaesth. 2010;105(Suppl 1):i97–107. doi: 10.1093/bja/aeq273 [DOI] [PubMed] [Google Scholar]
- 30.Tran TA, Williams LM, Bui D, Anthonisen C, Poltavskiy E, Szabo RM. Prospective pilot study comparing pre- and postsurgical CTSAQ and neuro-QoL questionnaire with median nerve high-resolution ultrasound cross-sectional areas. J Hand Surg Am. 2018;43(2):184e181–184 e189. doi: 10.1016/j.jhsa.2017.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raeissadat SA, Karimzadeh A, Hashemi M, Bagherzadeh L. Safety and efficacy of platelet-rich plasma in treatment of carpal tunnel syndrome; a randomized controlled trial. BMC Musculoskelet Disord. 2018;19(1):49. doi: 10.1186/s12891-018-1963-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senna MK, Shaat RM, Ali AAA. Platelet-rich plasma in treatment of patients with idiopathic carpal tunnel syndrome. Clin Rheumatol. 2019;38(12):3643–3654. doi: 10.1007/s10067-019-04719-7 [DOI] [PubMed] [Google Scholar]
- 33.Catapano MCJ, Borschel G, Alavinia SM, Robinson LR, Mittal N. Effectiveness of platelet-rich plasm injections for nonsurgical management of carpal tunnel syndrome: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2020;101:897–906. doi: 10.1016/j.apmr.2019.10.193 [DOI] [PubMed] [Google Scholar]
- 34.Liza Maniquis-Smigel KDR, Rosen HJ, Lyftogt J, Graham-Coleman C, Cheng A-L, Rabago. D. Analgesic effect of caudal 5% dextrose in water in chronic low back pain. A randomized controlled trial of epidural injection. Anesth Pain Med. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maniquis-Smigel L, Reeves KD, Rosen HJ, et al. Analgesic effect and potential cumulative benefit from caudal epidural D5W in consecutive participants with chronic low-back and buttock/leg pain. J Altern Complement Med. 2018;24(12):1189–1196. doi: 10.1089/acm.2018.0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malek N, Pajak A, Kolosowska N, Kucharczyk M, Starowicz K. The importance of TRPV1-sensitisation factors for the development of neuropathic pain. Mol Cell Neurosci. 2015;65(Mar):1–10. doi: 10.1016/j.mcn.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 37.Szolcsányi JLSZ, Sándor Z. Multisteric TRPV1 nocisensor: a target for analgesics. Trends Pharmacol Sci. 2012;33(12):646–655. doi: 10.1016/j.tips.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 38.Bertrand H, Kyriazis M, Reeves KD, Lyftogt J, Rabago D. Topical mannitol reduces capsaicin-induced pain: results of a pilot-level, double-blind, randomized controlled trial. PM R. 2015;7(11):1111–1117. doi: 10.1016/j.pmrj.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 39.Fujita SBM, Bohland M, Watts G, Watts AG, Donovan CM. Hypoglycemic detection at the portal vein is mediated by capsaicin-sensitive primary sensory neurons. Am J Physiol Endocrinol Metab. 2007;293(1):96–101. doi: 10.1152/ajpendo.00415.2006 [DOI] [PubMed] [Google Scholar]
- 40.MacIver MB, Tanelian DL. Activation of C fibers by metabolic perturbations associated with tourniquet ischemia. Anesthesiology. 1992;76(4):617–623. doi: 10.1097/00000542-199204000-00020 [DOI] [PubMed] [Google Scholar]
- 41.Evers S, Thoreson AR, Smith J, Zhao C, Geske JR, Amadio PC. Ultrasound-guided hydrodissection decreases gliding resistance of the median nerve within the carpal tunnel. Muscle Nerve. 2018;57(1):25–32. doi: 10.1002/mus.25723 [DOI] [PMC free article] [PubMed] [Google Scholar]