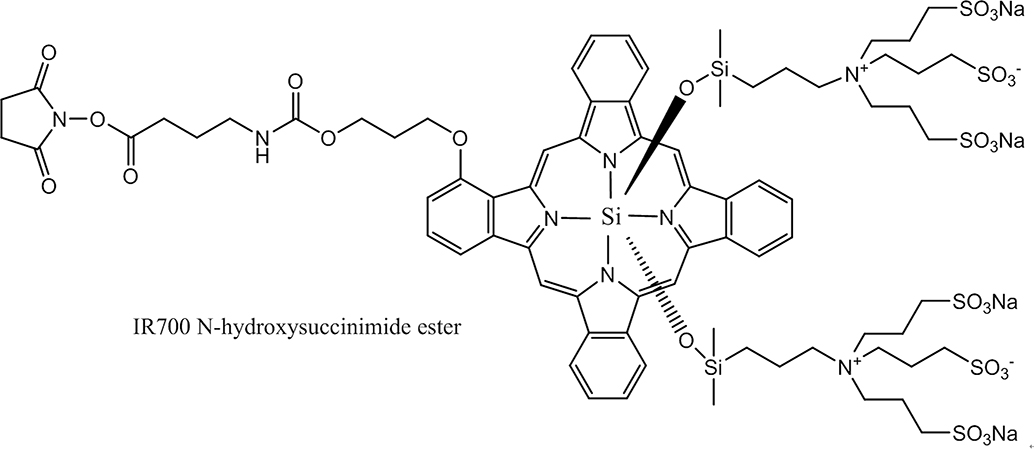
Figure 5.
The active ester-linker-IR700 dye structure, with the ester being used to couple to MAb lysine residues. Note that the aromatic, essentially flat, phthalocyanine ring is counter-balanced by the oxysilicopropyl-aminotripropyl sulfonic acid groups held above and below the aromatic core. The stereochemistry prevents pi stacking of more than one aromatic ring even at multi-substitution on an antibody, thereby alleviating both quenching and aggregation of the dye and MAb, respectively. The six appended sulfonic acid groups and the two tetra-amino groups render the dye zwitterionic at physiological pH and the resulting MAb-IR 700 conjugate highly water-soluble.