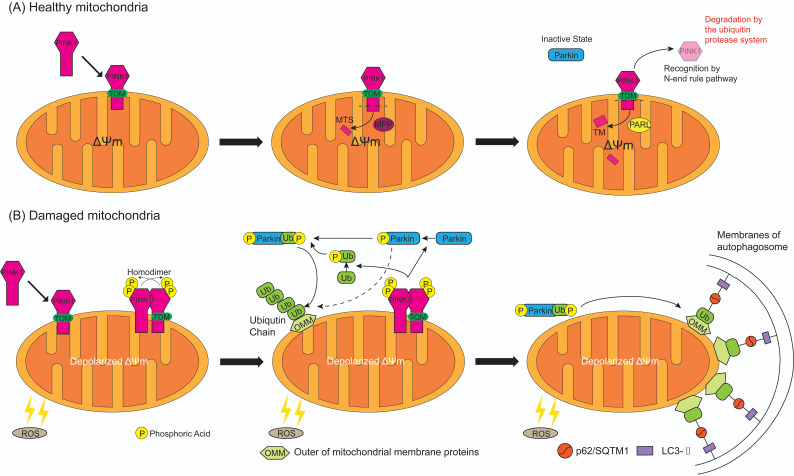
Figure 4.
The process of PINK1 and Parkin mediated mitophagy on healthy and damaged mitochondria. (A) While PINK1 is recruited to the healthy mitochondria, N-terminal mitochondrial targeting sequence (MTS) of PINK1 is translocated across the mitochondrial membranes through the translocase of the outer membrane (TOM), and is exposed to central matrix depending on ΔΨm. Then MTS is cleaved by mitochondrial processing peptidase (MPP) in the mitochondrial matrix and the TM segment of PINK1 is cleaved by presenilin-associated rhomboid-like protease (PARL) in the inner membranes. The rest of PINK1 with an instable amino acid at the N-terminal is released to cytosol, recognized by N-end rule pathway and rapid degraded by the ubiquitin protease system. As a result, Parkin keep in an inactive state in the cytosol. (B) When the mitochondria are damaged by the reactive oxygen species (ROS), ΔΨm is depolarized and MTS cannot reach into the matrix which results in the escape of PINK1 from MPP/PARL-induced processing and N-end rule pathway-dependent degradation, therefore PINK1 steadily binds with the TOM. Two molecules of PINK1 form a homodimer and are intermolecularly phosphorylated to become highly active. Then PINK1 induces the phosphorylation of Parkin and ubiquitin at Ser65. Activated Parkin combine with or without phosphorylated ubiquitin can ubiquitinate many OMM proteins, but phospho-ubiquitin binded to Parkin can maximally activate E3-ubiquitin ligase activity of Parkin. In consequence ubiquitinated OMM proteins bind to the autophagosome by either direct binding to the LC3-II embedded in the membrane of autophagosome, or indirectly through p62/ Sequestosome 1 (SQSTM1), which contains a LC3 interacting domain and can bind to LC3, then promotes mitophagy.