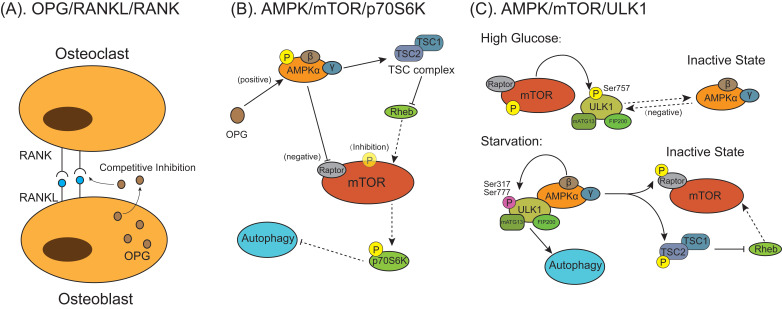
Figure 5.
The models for mammalian target of rapamycin (mTOR) behaviors on autophagic signaling pathway activation during osteoclast differentiation and formation. (A) Osteoprotegerin (OPG) is a decoy receptor for the RANKL and competitively inhibit osteoclasts differentiation and maturation through blocking the interaction between RANKL and RANK. (B) OPG inhibits osteoclastogenesis and bone resorption by enhancing autophagy through activating AMPK/mTOR/p70S6K signaling pathway. OPG activates AMP‐activated protein kinase (AMPK) and downstream tuberous sclerosis complex 2 (TSC2). Activated AMPK inhibits mTOR through either phosphorylating Raptor on mTOR or promoting TSC1/TSC2 complex formation to inhibit Ras homolog enriched in brain (Rheb), which can induce mTOR activation. Reduction of activated mTOR activate autophagy indirectly by nonactivated 70‐kDa ribosomal protein S6 kinase (p70S6K). (C) The AMPK/mTOR/UNC-51 like autophagy activating kinase 1 (ULK1) signaling pathway mediated autophagy is involved in the regulation of energy metabolism in osteoclastogenesis. Under high glucose condition, activated mTOR phosphorylates ULK1 at Ser757, which inhibits the interaction between ULK1 and AMPK, to suppress autophagy. Under glucose starvation conditions when energy supply is exhausted, activated AMPK phosphorylates TSC2 and Raptor, which inhibits the activation of mTOR, then signal transduction from AMPK to ULK1 is restored. AMPK phosphorylates and activates ULK1 at multiple residues (Ser317 and Ser777), the activated ULK1/mATG13/FIP200 protein kinase complex lead to autophagy.