Figure 1. Study Design of the ER+ AKT1-Mutant Breast Cancer Patient Cohorts.
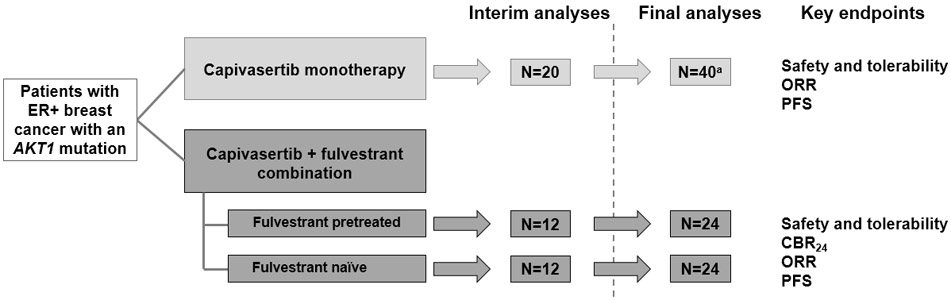
The breast cancer cohorts were part of a larger open-label, multipart, Phase I study of the first-in-human evaluation of oral capivasertib in patients with advanced solid malignancies. These Phase I expansion cohorts were non-randomized; the monotherapy cohort enrolled first, followed by the combination therapy cohort. Protocol-specified analyses planned for each study part: For monotherapy, analyses were planned after 20 patients were followed up for 12 weeks/withdrawn from the study. For combination therapy, interim analysis was planned after 12 patients in each cohort were followed up for 24 weeks/withdrawn from the study, and final analysis was planned after up to 24 patients in total in each cohort were followed up for 24 weeks/withdrawn from the study. aUp to 120. CBR24, clinical benefit rate at 24 weeks; ER, estrogen receptor; ORR, objective response rate; PFS, progression-free survival.
