Figure 1.
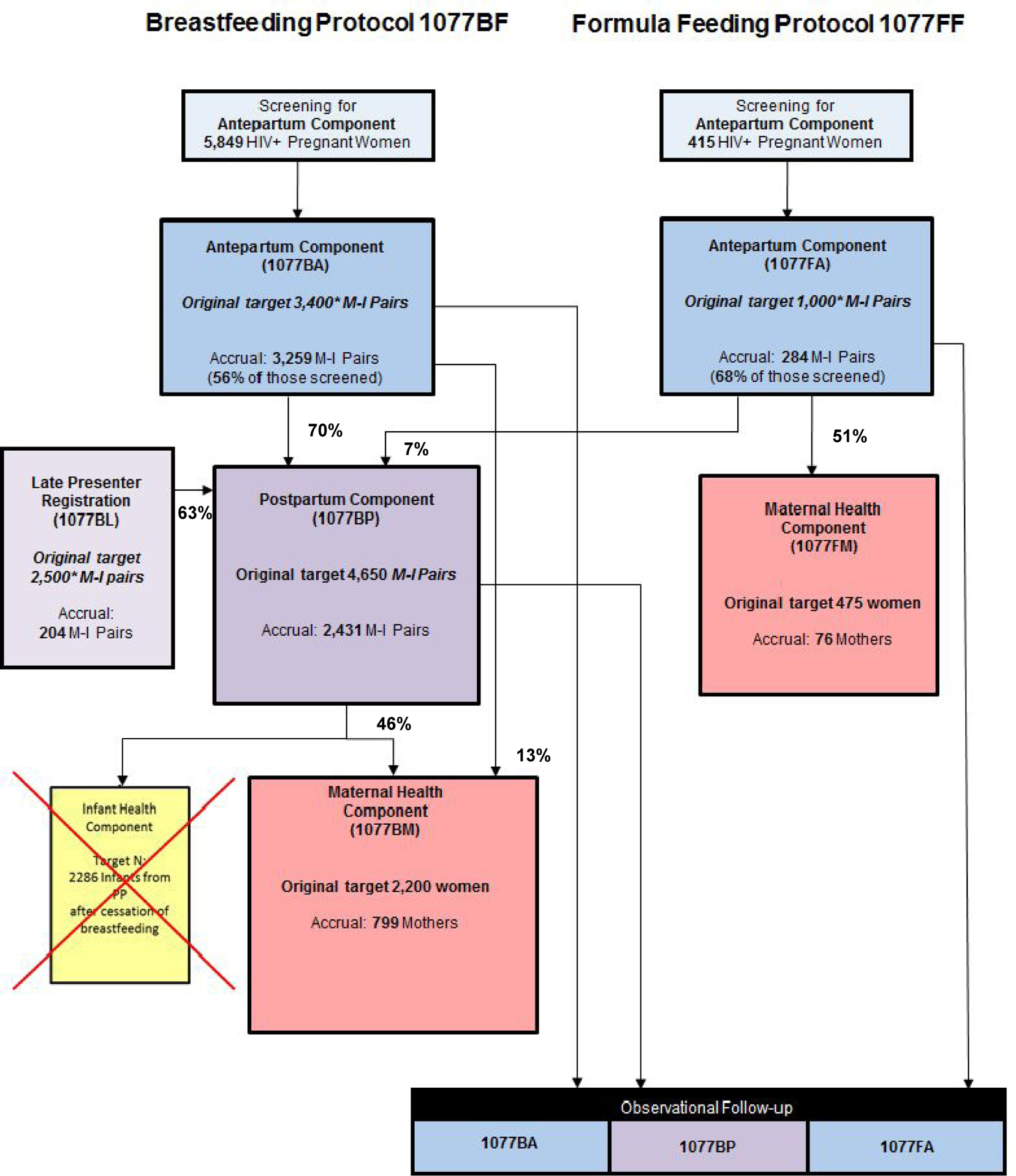
PROMISE target and actual accrual and transition rates
1077BF: PROMISE Breastfeeding protocol; enrolled women who intended to breastfeed their infants
1077FF: PROMISE Formula-feeding protocol; enrolled women who intended to formula-feed their infants
Component: one of the randomizations in the 1077BF or 1077FF protocols
* Target initial enrollment in PROMISE (in italics).
Originally, the 1077BF protocol also had a fourth randomization for HIV-uninfected infants who ceased breastfeeding prior to age 12 months, to assess the efficacy and safety of continued cotrimoxazole prophylaxis versus placebo for the prevention of infant mortality and morbidity following breastfeeding cessation. This Infant Health component however, did not open to enrollment due to a change in WHO guidelines to recommend breastfeeding of HIV-exposed infants beyond age 12 months.
It is important to note that the overall number of unique mother-infant pairs in PROMISE is much less than the sum of the component sample sizes. This is because 1077BF had only two points of entry (Antepartum and Late Presenters) and 1077FF had only one point of entry (Antepartum); the remaining PROMISE components would only enroll women and/or infants who participated in one of these initial PROMISE components. Transition and non-enrollment rates were calculated among the eligible MI pairs per component and protocol.
