Scheme 2.
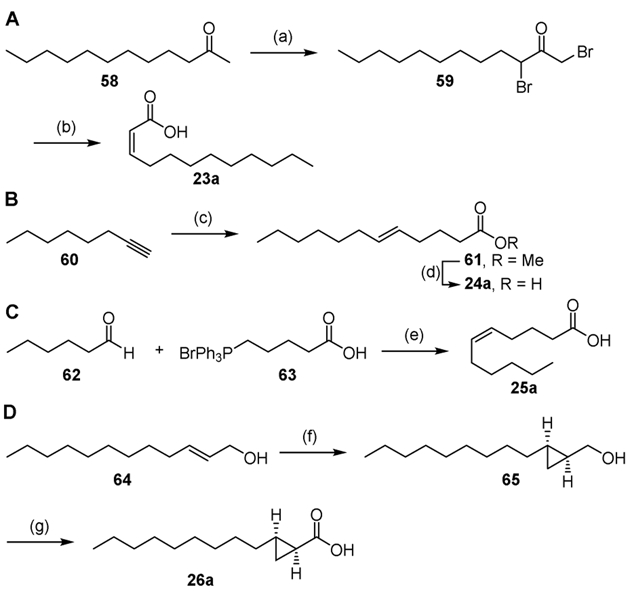
Synthesis of acids 23a–26a. Reaction conditions: (a) HBr (aq), Br2, 99%; (b) NaOH (aq), then HCl (aq), 87%; (c) Cp2ZrHCl, Pd2(dba)3, LiBr, NMP/THF, then ethyl 4-bromobutyrate, 79%; (d) LiOH, MeOH/H2O, 70%; (e) NaHMDS, THF, 40%; (f) (4R,5R)-2-butyl-N,N,N′N′-tetramethyl-1,3,2-dioxaborolane-4,5-dicarboxamide, Zn(CH2I)2, CH2Cl2, 66%; (g) CrO3, H2SO4, H2O/acetone, 75%.
