Extended Data Figure 11. EedKO mouse ESC differentiation recapitulates many features of the in vivo mutant phenotype.
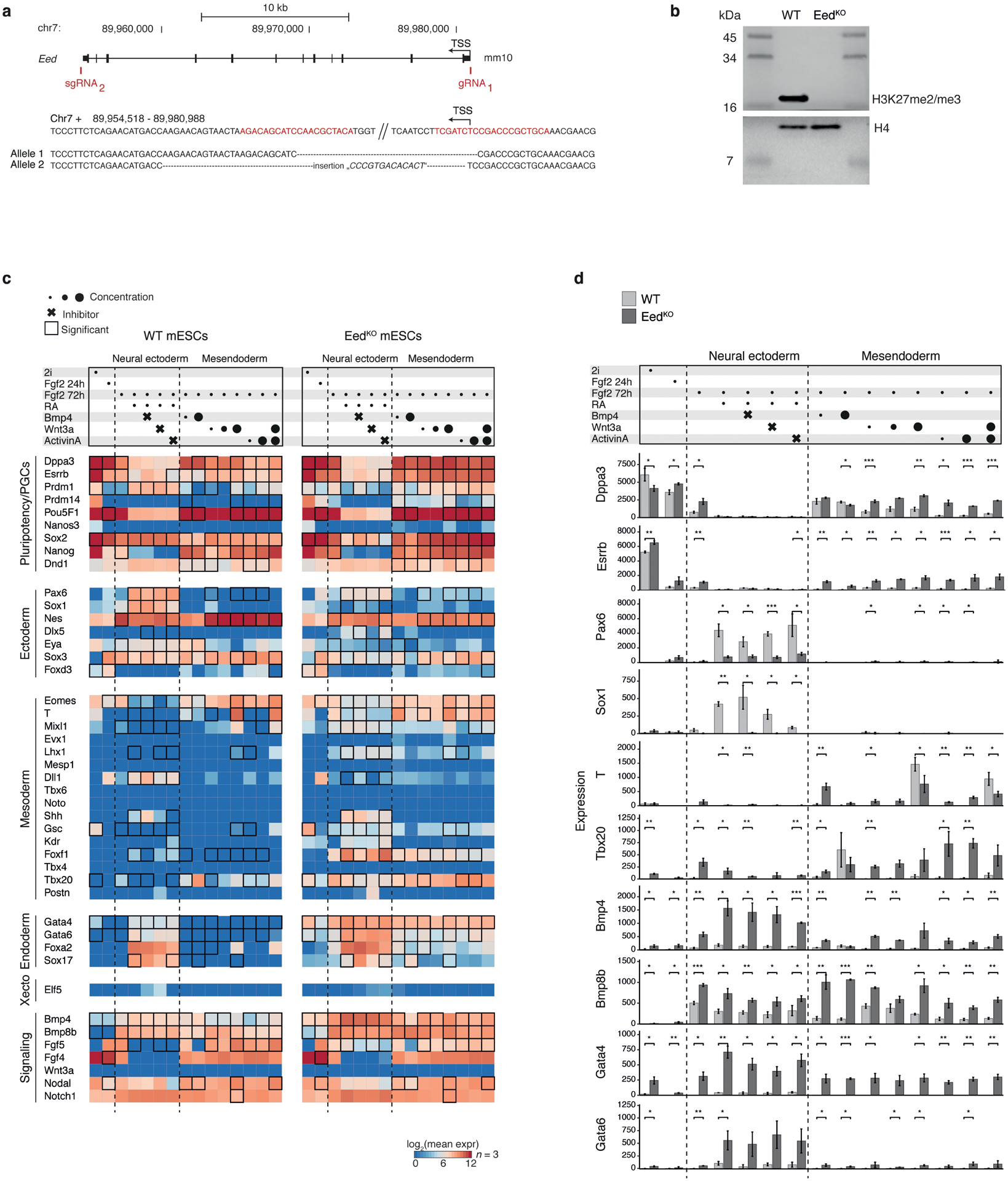
a. Generation of a homozygous EedKO mESC line. The Eed gene was deleted in V6.5 mESCs by simultaneous Cas9-targeting of flanking sequences (red) to create a >20 kb deletion. Sanger sequencing confirmed complete deletion by non-homologous end joining for both alleles (sequences aligned to chromosomal sequence above with dashes for missing nucleotides).
b. Western blot of histone extracts for H3K27me3 confirms homozygous Eed deletion and depletion of H3K27 trimethylation. Histone 4 served as loading control.
c. Transcript counts of 44 genes associated with pluripotency, early germ layers, and the germline74 over directed differentiation experiments from EedKO mESCs. WT and EedKO mESCs were maintained in conditions supporting a naïve, inner cell mass-like state (2i), then subjected to low concentrations of bFGF for 24 h followed by culture in neural ectodermal and mesendodermal inducers for an additional 48 h. Top: The combination of signaling molecules and/or inhibitors used. Concentration ranges are indicated by circle diameter and small molecule inhibitors by crosses. Inhibitors were included to promote neural ectodermal gene induction by counteracting competing pathways. 12 ng/ml bFGF; 0.25 μM RA; 5 and 500 ng/ml BMP4; 10, 100, 1000 ng/ml WNT-3A; 10 and 1000 ng/ml ACTIVIN A; 0.5 μM BMP4 pathway inhibitor LDN-193189; 3.3 μM Wnt pathway inhibitor XAV939; 10 μM TGF-β/Activin/NODAL pathway inhibitor SB431542. Bottom: Heatmap of log2-transformed molecule counts (red being highly expressed) for WT and EedKO, separately. Black tile frames indicate significant changes between WT and KO. During differentiation, many pluripotency factors associated with the germline remain expressed in EedKO mESCs, especially within mesendodermal supporting conditions. Many mesodermal genes are also induced in EedKO in neural ectodermal supporting conditions. Retinoic Acid (RA) treatment directs a fraction of WT mESCs to an extraembryonic endodermal fate75. This appears to be favored in EedKO mESCs, where many Xendo-associated genes are particularly sensitive to RA. Finally, many regulators of the endoderm, early mesendoderm and extraembryonic mesoderm, such as Gata4, Tbx20 and Bmp4, are broadly expressed in EedKO mESCs already in 2i/LIF. n = 3 experimental replicates profiled with the PlexSet assay on the NanoString nCounter SPRINT instrument. Significance (two-sided t-test) was tested for genes in conditions where at least one of the two cell lines produces a minimum average of 50 counts above background.
d. Barplots of normalized, absolute Nanostring molecule counts for the genes and conditions presented as fold change in Fig. 4f. Mesodermal genes exhibit some responsiveness to exogenous signals and can be induced to different degrees under supportive conditions (BMP4 and WNT3A). However, many are also more highly expressed without these stimuli, particularly genes associated with posterior, extraembryonic mesodermal fates. Raw counts were normalized based on the expression of four housekeeping genes and a conservative background subtraction to account for the potential of false negative signals. Two-sided t-test, * <0.05, ** <0.01, ***<0.001, bars show the mean of n = 3 experimental replicates and error bars represent standard deviation. P-values are provided in the Source Data file Nanostring_pval.tsv.
