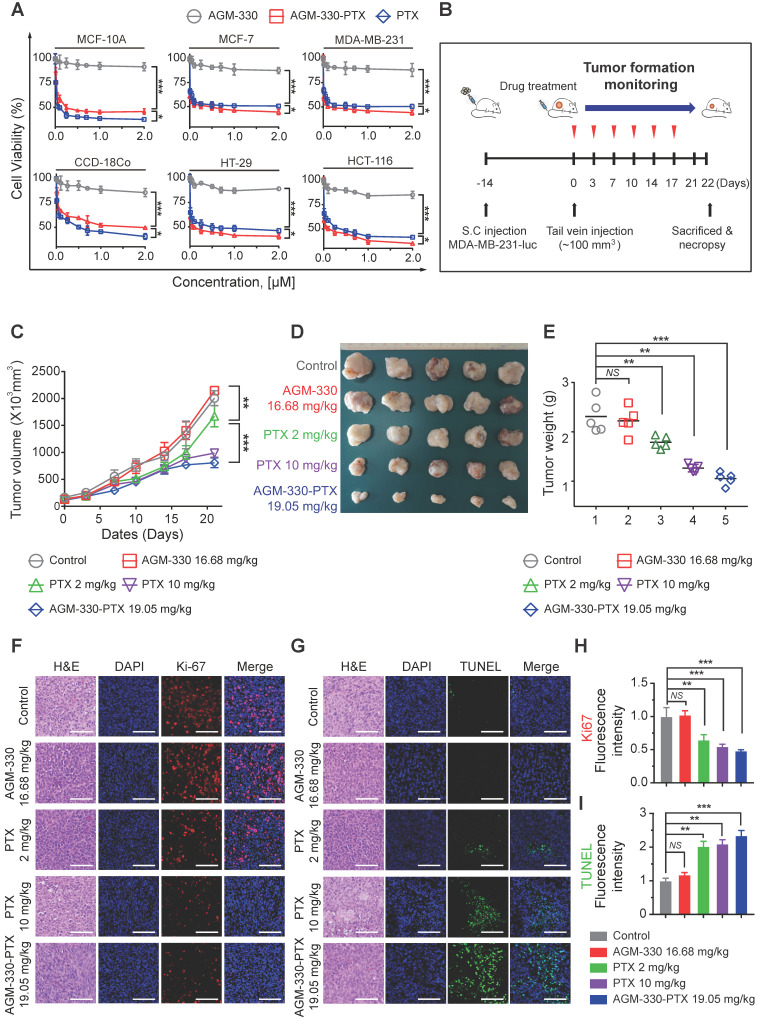
Figure 5.
AGM-330 led to increased therapeutic efficacy of PTX in vivo. A, Cell proliferation inhibition by AGM-330-PTX treatment was determined for 48 h by the WST assay in multiple breast and colorectal cancer cells and in normal cells. B, Schematic representation of the experimental protocol as described in the materials and methods section. Anesthetized 6‑week-old male NPG mice were inoculated with a 1:1 mix of Matrigel and 1 × 106 MDA-MB-231-luc cells into the mammary fat pad. When the volume of primary tumors reached approximately 100 mm3, tumor-bearing mice were treated with vehicle (PBS), PTX (2 or 10 mg/kg), AGM-330 (16.68 mg/kg, molar equivalent to 2 mg/kg of PTX), or AGM-330-PTX (19.05 mg/kg, molar equivalent to 2 mg/kg of PTX; n = 5/group). C-E, The therapeutic effect of AGM-330-PTX was evaluated in breast cancer xenograft models. C, Primary tumor volume was measured twice a week until the day of sacrifice. The primary tumor volume was calculated by the formula volume (mm3) = (length (mm)) × (width (mm))2 × 0.5. D, On the day of sacrifice, all primary tumors were isolated, and (E) the primary tumor weights were evaluated. F-I, Immunohistochemistry (IHC) sections of representative tumors with Ki-67 (F,H) and TUNEL (G,I) staining. Nuclei were stained with DAPI (blue). Scale bar, 50 µm. Bar graphs represent the mean ± SD, and statistical analyses were performed by one-way ANOVA with Dunnett's multiple comparison. *, **, and *** indicate P < 0.05, P < 0.01, and P < 0.001, respectively.