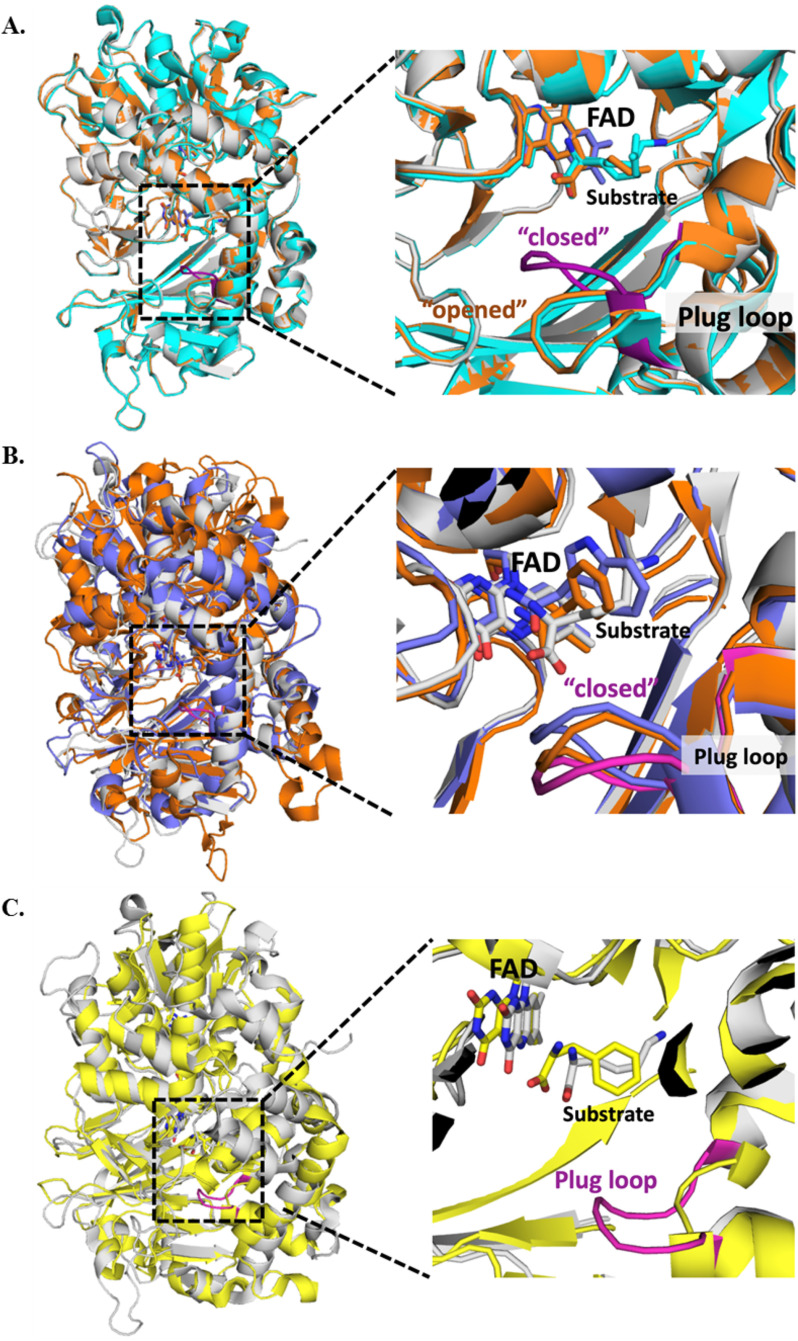
Figure 9.
The crystal structures of l-LOX/MOG showing the plug loop with eight residues from Val-228 to Trp-235 (VGFGTGGW). A, l-LOX/MOG with l-lysine bound (gray), l-ornithine bound (orange), and l-arginine bound (cyan). The plug loop of the l-lysine–bound structure is shown in magenta. B, structure of l-LOX/MOG with l-lysine bound (gray) and plug loop (magenta) is aligned with PAO from Pseudomonas sp. P-501 with l-phenylalanine bound (purple) and TMO from P. savastanoi with IAM bound (orange). These two enzymes can also catalyze decarboxylation of their imino acids. C, the structure of l-LOX/MOG with l-lysine bound (gray) is aligned with the structure of l-AAO from C. rhodostoma with l-phenylalanine bound (yellow). l-AAO cannot catalyze the decarboxylation of imino acids, and the alignment does not show any plug loop in the l-AAO structure.