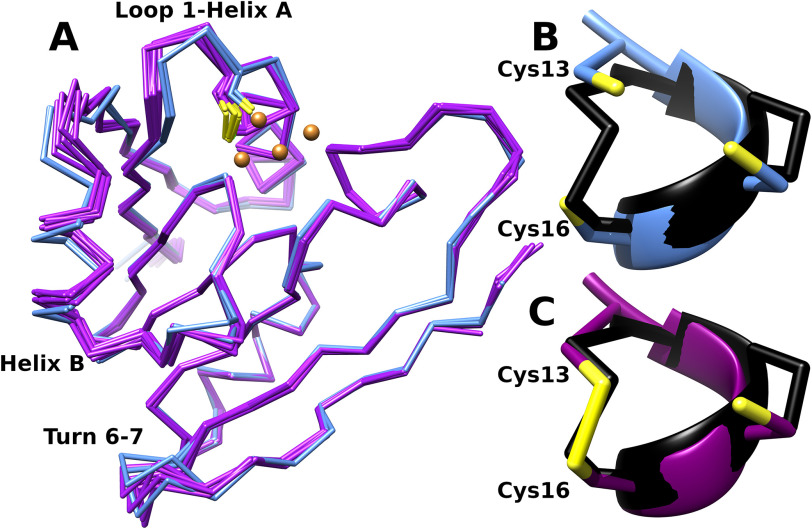
Figure 7.
Structural consequences of disulfide formation. A, superposition of the Cα traces of the 2 form I models (blue) representing the reduced state and 6 form II models (purple) representing the oxidized state. Disulfide formation between sites 13 and 16 is associated with displacement of helix B and a bending of the loop between strand 1 and helix A relative to the reduced state. The loop between strands 6 and 7 also shows structural variability, but there does not appear to be a discrete difference in this region between the two crystal forms. B and C, detail of residues 13 to 16 showing reduced thiolates on Cys 13 and 16 (form I, chain A) (B) and disulfide (form II, chain A) (C). These are superimposed on the corresponding residues (C32-I38, in black) from the structure of oxidized E. coli thioredoxin (PDB code 2TRX [69]).