Figure 4.
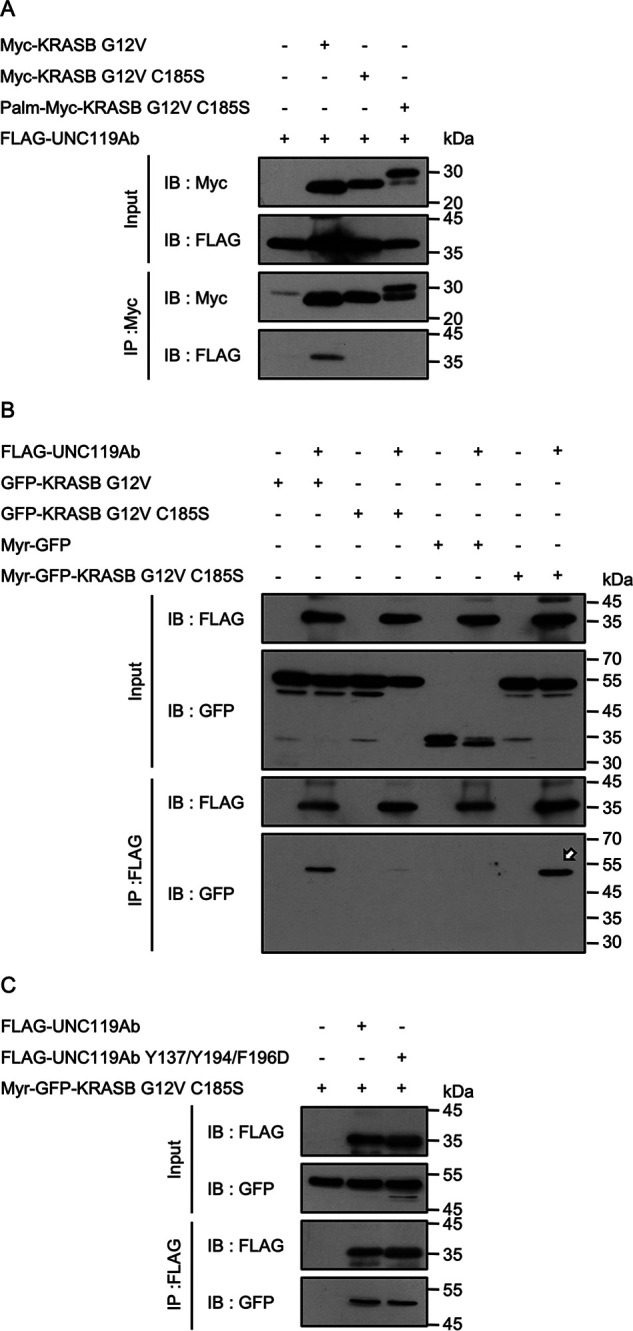
The effect of lipid modification on the interaction between KRASB and UNC119Ab. A, Myc-KRASB G12V, Myc-KRASB G12V C185S, and Palm-Myc-KRASB G12V C185S were coexpressed with FLAG-UNC119Ab in HEK293FT cells. Immunoprecipitation was performed with anti-Myc antibody. UNC119Ab coimmunoprecipitated with neither Myc-KRASB G12V C185S nor Palm-Myc-KRASB G12V C185S. B, FLAG-UNC119Ab was coexpressed with GFP-KRASB G12V, GFP-KRASB G12V C185S, Myr-GFP, and Myr-GFP-KRASB G12V C185S in HEK293FT cells and was immunoprecipitated. GFP-KRASB G12V and Myr-GFP-KRASB G12V C185S coimmunoprecipitated with FLAG-UNC119Ab (arrow), whereas GFP-KRASB G12V C185S or Myr-GFP was not. C, Myr-GFP-KRASB G12V C185S coimmunoprecipitated with FLAG-UNC119Ab Y137/Y194/F196D, which excludes the possibility that the artificial myristoylated sequence binds to the hydrophobic pocket. Four experiments for panel A and three experiments for panels B and C were performed by two members.
