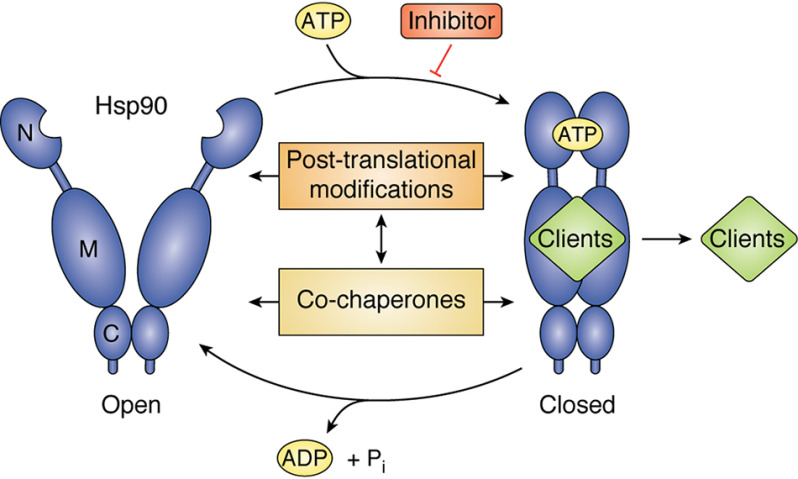
Figure 1.
The Hsp90 chaperone cycle. Hsp90 begins its chaperone cycle in an open conformation that is dimerized only at the C-domain. ATP binding and an ordered series of conformational changes allow it to adopt a closed conformation, which is N-terminally dimerized. Upon ATP hydrolysis, Hsp90 returns back to the open conformation and is ready to begin another chaperone cycle. This allows for the activation of client proteins. This cycle is tightly regulated by co-chaperone proteins as well as PTMs, and Hsp90 inhibitors can also modulate the chaperone cycle.