Figure 3.
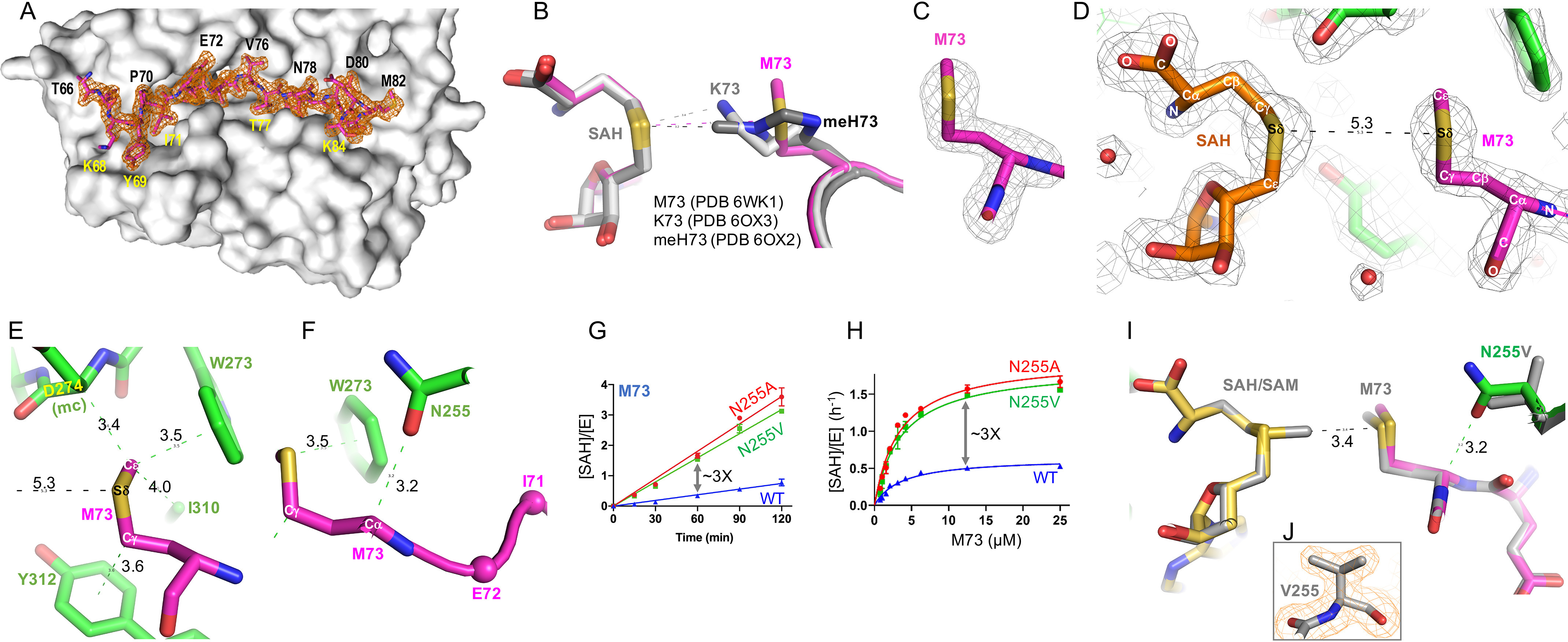
Structure of SETD3 in complex with Met73 in the active site. A, a surface representation of SETD3 (colored gray) accommodation of Met73 peptide in a long surface groove. Omit electron density for ordered residues 66–84 (in stick model) is contoured at 3σ above the mean. B, superimposition of SETD3 in complex with Met73, Lys73, and methylated His73. C, omit electron density (Fo − Fc) for Met73 contoured at 4σ above the mean. D, the Met73 side chain and the methionine moiety of SAH is nearly symmetric to each other by a rotation of 180°. E, the nonpolar S-methyl thioether side chain is in contact with aromatic and hydrophobic residues. F, a C–H···O hydrogen bond is formed between the main chain Cα atom of Met73 of actin and side chain of Asn255 of SETD3. G and H, SETD3 N255V and N255A mutants have high activity on Met73. I, superimposition of SETD3 (N255) (PDB code 6WK1) and its mutant N255V (PDB code 6WK2) in complex with actin Met73 peptide. J, electron density (2Fo − Fc) for Val255 contoured at 2.0σ above the mean.
