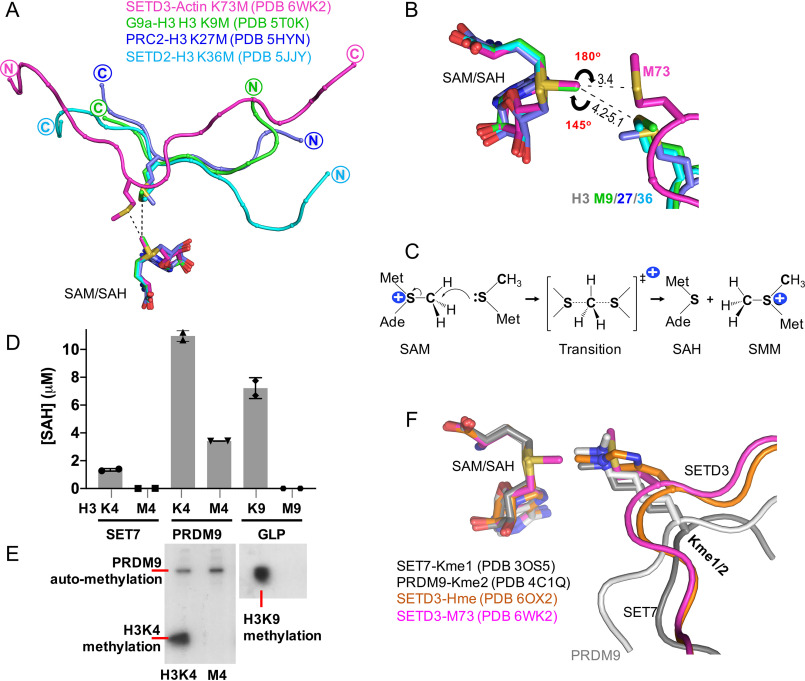
Figure 4.
Comparison of active-site configuration for histone SET domain proteins and SETD3. A, superimposition of SETD3, G9a, PRC2, and SETD2 in complex with methionine-substituted peptide substrates. B, a linear arrangement comprising the Sδ atom of Met73, the methyl group, and the leaving thioester group of SAM. C, the Sn2 reaction mechanism by SAM-dependent methylation. D, SET7, PRDM9, and GLP are not active on methionine-substituted histone H3 peptides. E, PRDM9 is automethylated (see Fig. S2). F, superimposition of SETD3 in complex with methylated histidine, SET7 with monomethylated lysine, and PRDM9 with dimethylated lysine substrates.