Figure 4.
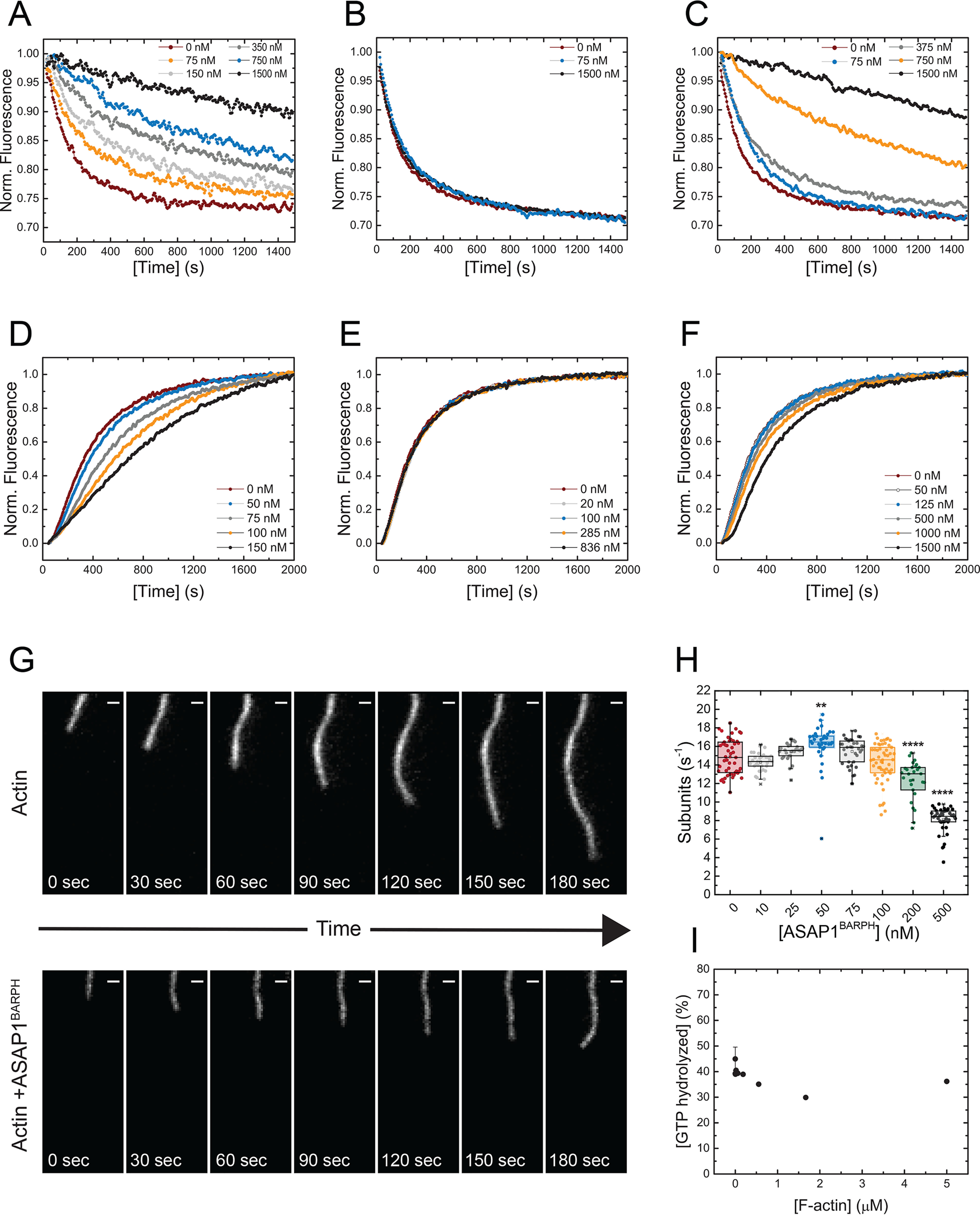
ASAP1BARPH stabilizes F-actin bundles and decreases the spontaneous polymerization of G-actin to F-actin. A, increasing concentrations of ASAP1BARPH protect F-actin bundles against depolymerization. ASAP1PH (B) and VTail (C) were used as negative and positive controls, respectively. A premixing concentration of 4 μm F-actin and the indicated concentrations of ASAP1BARPH, ASAP1PH, or VTail was used. The representative plotted fluorescence intensities are normalized to 1 for the initial fluorescence at t = 0 of each sample shown in panels A–C. D, ASAP1BARPH decreases the spontaneous polymerization of 3.5 μm G-actin (10% pyrene labeled) to F-actin. ASAP1PH does not affect actin polymerization (E), whereas VTail slightly decreases the actin polymerization rate under the same experimental conditions (F). Representative data are shown. G, montage of images showing the elongation of individual actin filaments alone (top) or in the presence of 500 nm ASAP1BARPH (bottom). Time interval, 30 s; scale bar, 1 μm. (H) Actin elongation rates as a function of ASAP1BARPH concentration (n = 28–55). ****, p < 0.0001, and **, p < 0.01, using one-way ANOVA with Dunnett's multiple-comparison test. I, F-actin does not influence the enzymatic GAP activity of ASAP1BARPZA in vitro (n = 2).
