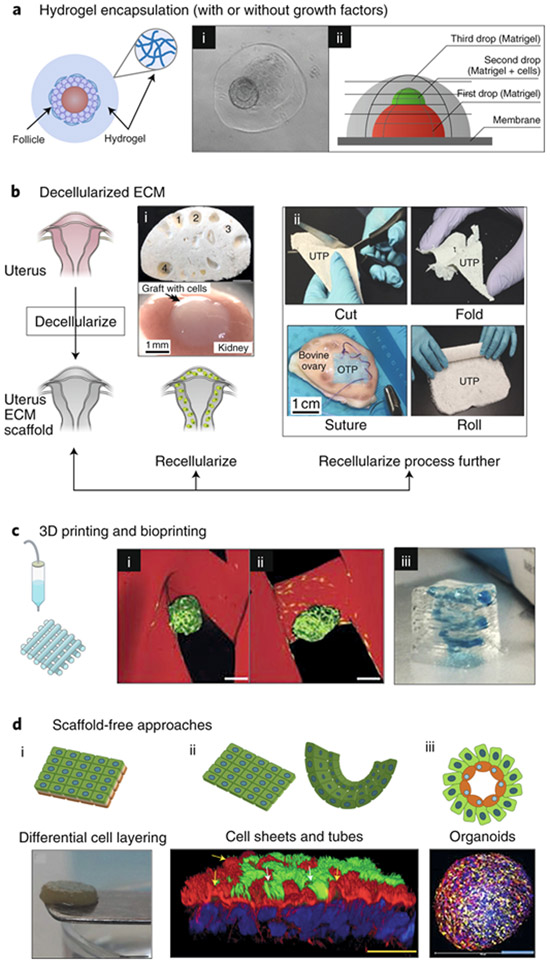
Fig. 4: Strategies for the use of engineered biomaterials in reproductive science and medicine.
a, Hydrogel encapsulation enables the in vitro culture of ovarian follicles and testicular cells in a manner that mimics their in vivo architecture. (i) 3D structure of the ovarian follicle (arrow) was maintained within collagen–alginate beads55. (ii) A three-layer gradient system consisting of testis-cell-containing Matrigel placed between two layers of cell-free Matrigel186. The gradient of cells promotes cellular reorganization into functional testicular organoids. b, Decellularization techniques for ovarian, uterine and testis tissues, for both basic research and transplantation. Following decellularization, tissue scaffolds can be (i) directly recellularized or (ii) further processed into other biomaterials. (i) Transplanted decellularized bovine ovary scaffolds (top) seeded with murine ovarian cells (bottom) to restore puberty in ovariectomized mice64. The numbers indicate visible pores where large follicles resided and that are maintained following decellularization. (ii) Decellularized composite materials from a variety of organ sources. UTP, bovine uterus ECM; OTP, bovine ovary ECM; CTP, bovine collagen88. The composite materials are ideal surgical materials as they can be cut, folded, sutured and rolled, and can support cell adhesion and survival. c, 3D printing and bioprinting provide precise control in the construction of engineered scaffolds for reproductive tissues. (i) 3D-printed bioprosthetic ovaries restore both fertility and endocrine function after transplantation into ovariectomized mice20. A tortuous pore network (i) better maintains the ovarian-follicle (green) architecture within the 3D-printed gelatin scaffold (red) compared to a grid-like 90° pore network (ii). Scale bars, 100 μm. (iii) Bioprinted model of the placenta by using human trophoblast cells to study pre-eclampsia96. The 3D-printed spiral (blue) resembles the maternal spiral arteries, which trophoblasts invade during normal placentation. d, Scaffold-free approaches, including self-assembling organoids, cell sheets and differential cell layering, have been used to create 3D models of reproductive tissues. (i) Gross appearance of a disk-shaped, scaffold-free 3D cervical stromal equivalent, onto which primary human cervical cells were seeded so as to generate a 3D-human ectocervix model102. (ii) Bovine oviduct epithelial cells self-assembled to form floating vesicles with outward facing actively beating cilia (green; indicated by white arrows). Actin filaments appear in red; nuclei appear in blue118; yellow arrows indicate primary cilia and actin-rich secretory protrustions. (iii) By using the hanging-drop method, testicular organoids composed of all testicular cell subtypes were created. Spermatogonial stem cells appear in blue, Sertoli cells in yellow and Leydig cells in red108.