Figure 4: AMG510+cetuximab combination is effective in KRAS G12C mutant patient-derived models.
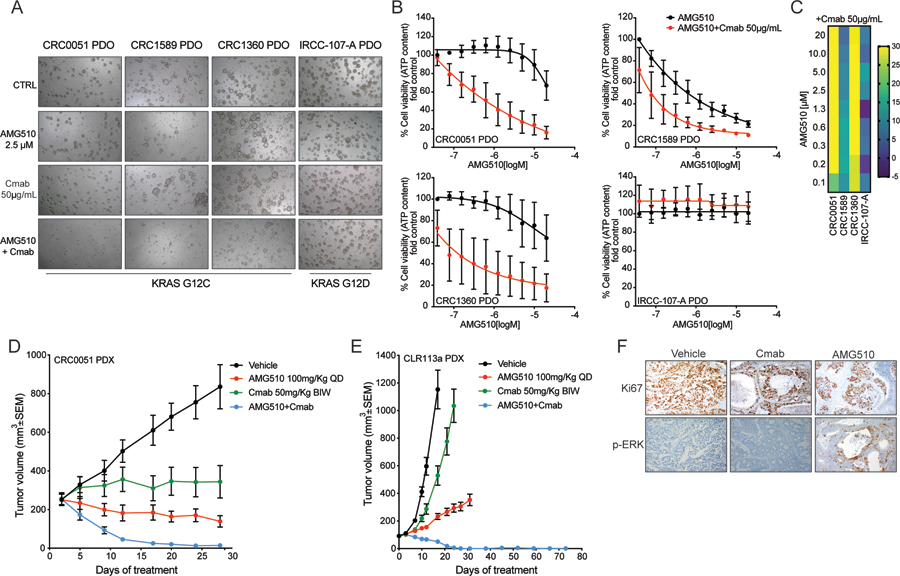
(A) Bright-field microscopy images of patient-derived organoid (PDO) treated with vehicle, AMG510, cetuximab and the combination 7 days after treatment. (B) ATP content quantification with CellTiterGlo assay. (C) Synergy score. Heatmap of excess over the Bliss (Bliss score) for AMG510-Cetuximab combination. (D) CRC0051 KRAS G12C mutant CRC patient-derived xenografts (PDXs) were treated with vehicle alone, AMG510 100mg/kg oral BID, cetuximab 50 mg/kg intraperitoneal twice a week (BIW), or the combination of the 2 drugs at the same doses. Error bars represent SEM (5 animals per group) (E) CLR113a KRAS G12C mutant CRC patient-derived xenografts (PDXs) were treated with vehicle alone, AMG510 100mg/kg oral BID, cetuximab 50 mg/kg intraperitoneal twice a week (BIW), or the combination of the 2 drugs at the same doses. Error bars represent SEM (5 animals per group). (F) Ki67 and phospho-ERK immunohistochemical staining of the CLR113a PDX samples collected at the end of treatment from vehicle, cetuximab and AMG510 treated arms. Ki67 IHC intensity of 97% with no necrosis in vehicle treated mice, 92% with necrosis in cetuximab treated mice, and 83% with necrosis in AMG510 treated mice.
