Figure 1. In vitro selection of hPD-L1 binding peptides by mRNA display.
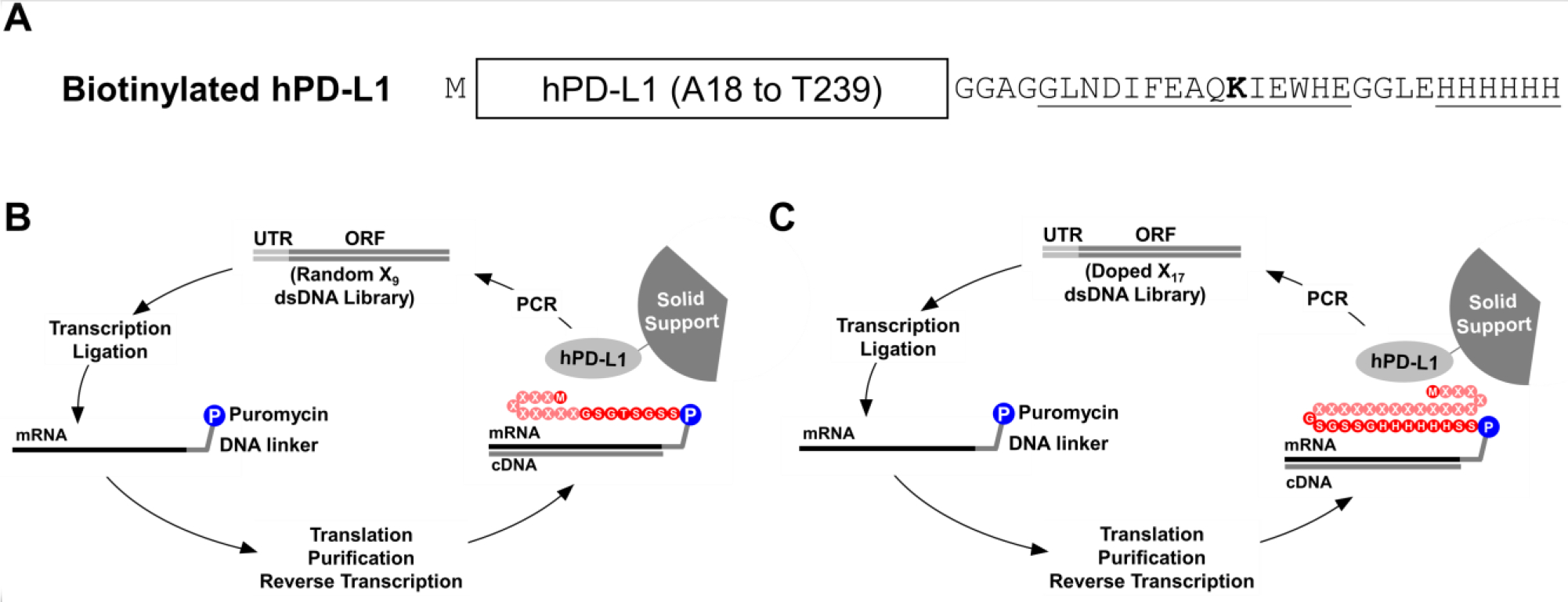
A) Biotinylated hPD-L1 target was expressed in E. coli and consisted of the extracellular domain of hPD-L1 (from A18 to T239), an N-terminal methionine, and a C-terminal hexahistidine tag and an Avi Tag, which was site-specifically biotinylated on the ε-amino group of lysine (bold) using the BirA biotin ligase. B) The initial selection was performed using a double stranded DNA library (Random X9 dsDNA Library) where the DNA was consisted of a 5’ untranslated region (UTR), a T7 promoter, a ΔTMV translation enhancer, and an open reading frame (ORF). Randomized positions are denoted with an “X” and could be any of the 20 natural amino acids. The DNA library was transcribed into RNA and ligated to a puromycin-bearing DNA linker. The template was subsequently translated, purified, and reverse transcribed to generate the mRNA-peptide fusion library, which was incubated with immobilized hPD-L1. The enriched library was amplified by PCR to generate the DNA library for the next cycle. C) A secondary selection was performed with a doped library (Doped X17 dsDNA Library) where residues denoted by an X were partially doped with the residues in hPD-L1 signal peptide.
