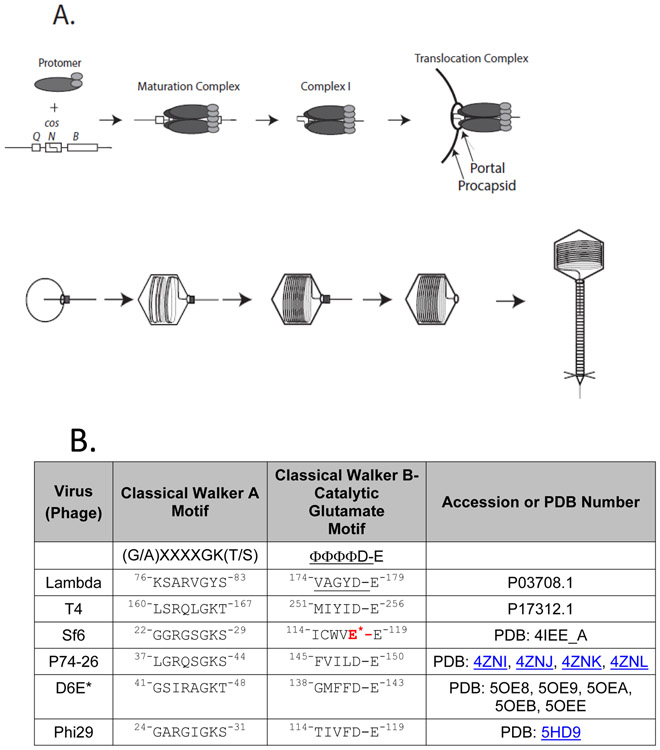
Figure 1. Model of lambda genome packaging.
A-Upper: Packaging initiation. The terminase protomer is a tight association of two TerSλ subunits and one TerLλ subunits. In the N-terminal domain (NTD) of TerSλ is a winged helix-turn-helix DNA binding motif and the TerSλ dimerization interface. The TerSλ C-terminal domain (CTD) includes a functional domain for interacting with TerLλ’s N-terminus. TerLλ consists of two globular domains connected by a linker. The TerLλ NTD contains the DNA packaging ATPase and the CTD contains the maturation endonuclease. Four protomers form into a ring-like structure at the cos site of a lambda genome concatemer, assembling the maturation complex. Maturation complex assembly involves interactions of TerSλ with cosB, and the TerLλ endonuclease with cosN, and requires ATP and/or IHF. Following duplex nicking by TerLλ and ejection of the upstream, cosQ-containing DNA end yields the post-cleavage complex (Complex I). Complex I docks at the portal of an empty procapsid, producing the packaging motor complex. Complex I docking involves an interaction between the TerLλ C-terminal tether and the portal protein. Docking activates the packaging ATPase, which powers translocation of DNA into the procapsid shell. We note that the stoichiometry of the protomer subunits bound to the procapsid, along with the subunit arrangement in the maturation complex, remain speculative at this time. A-Lower: Translocation and virion assembly. Progression of translocation is shown proceeding left to right. When ~30% of the DNA has been translocated, procapsid expansion is triggered, and translocation proceeds until the next cos along the concatemer is encountered. Interactions between the translocating terminase and cosQ and cosN lead to nicking of the downstream cos, and terminase undocking. Addition of gpW and gpFII to the portal is followed by tail attachment, generating a mature virion. B. ATPase motif sequences. Walker B motif and catalytic glutamate ASCE sequences are given for TerLs discussed in this paper. Beginning and ending residue numbers are given in superscripts. *Note that TerLSf6 contains a deviant glutamate (red) rather than the canonical aspartate found in most ASCE Walker B sequences