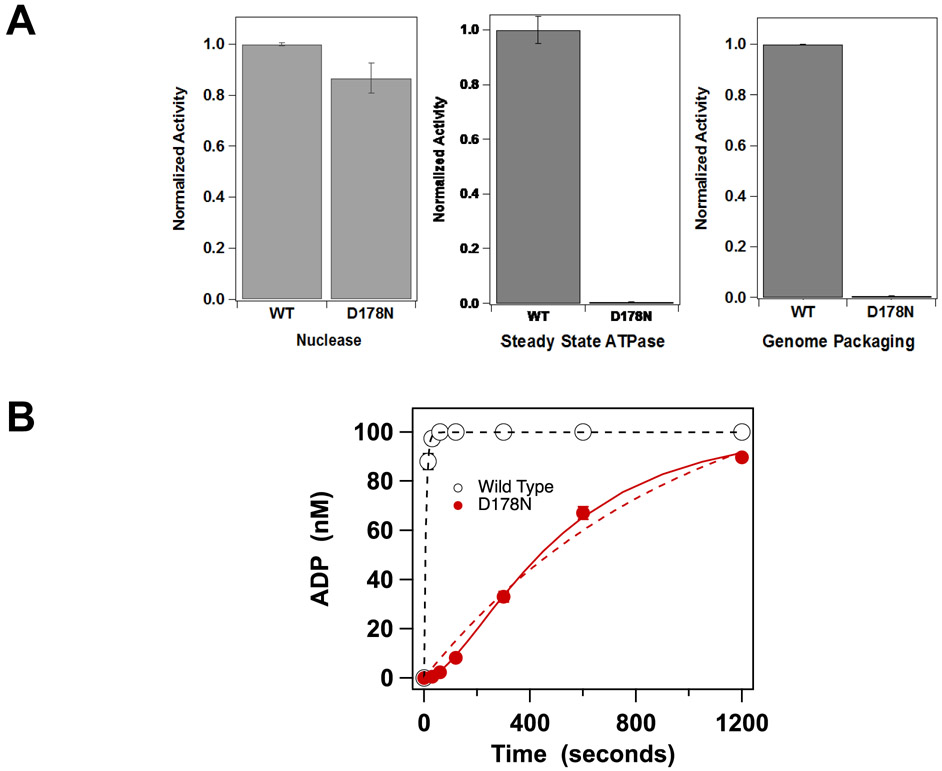
Figure 5. Catalytic Activity of TerLλ-D178N terminase.
cos-cleavage nuclease, DNA packaging and steady-state ATPase activities were performed as described in Materials and Methods. Each bar represents the average of three separate experiments with error-bars indicating the standard errors in the mean. (B) Single turnover ATP hydrolysis was performed as described in Materials and Methods; the reaction was initiated by the addition of enzyme. The reaction time courses for WT (○) is well described by a single-turnover, monophasic exponential model (solid line). In contrast, TerLλ-D178N displays a significant lag phase (●), which is poorly described by the simple model (see text; dashed red line). The mutant data are better described by the three-state model that includes a slow step prior to catalysis (Equation 1, solid red line). The kinetic constants derived from non-linear regression analysis of each data set is presented in Table 3.