Figure 7. Model of ABCC4 function in SHH signaling.
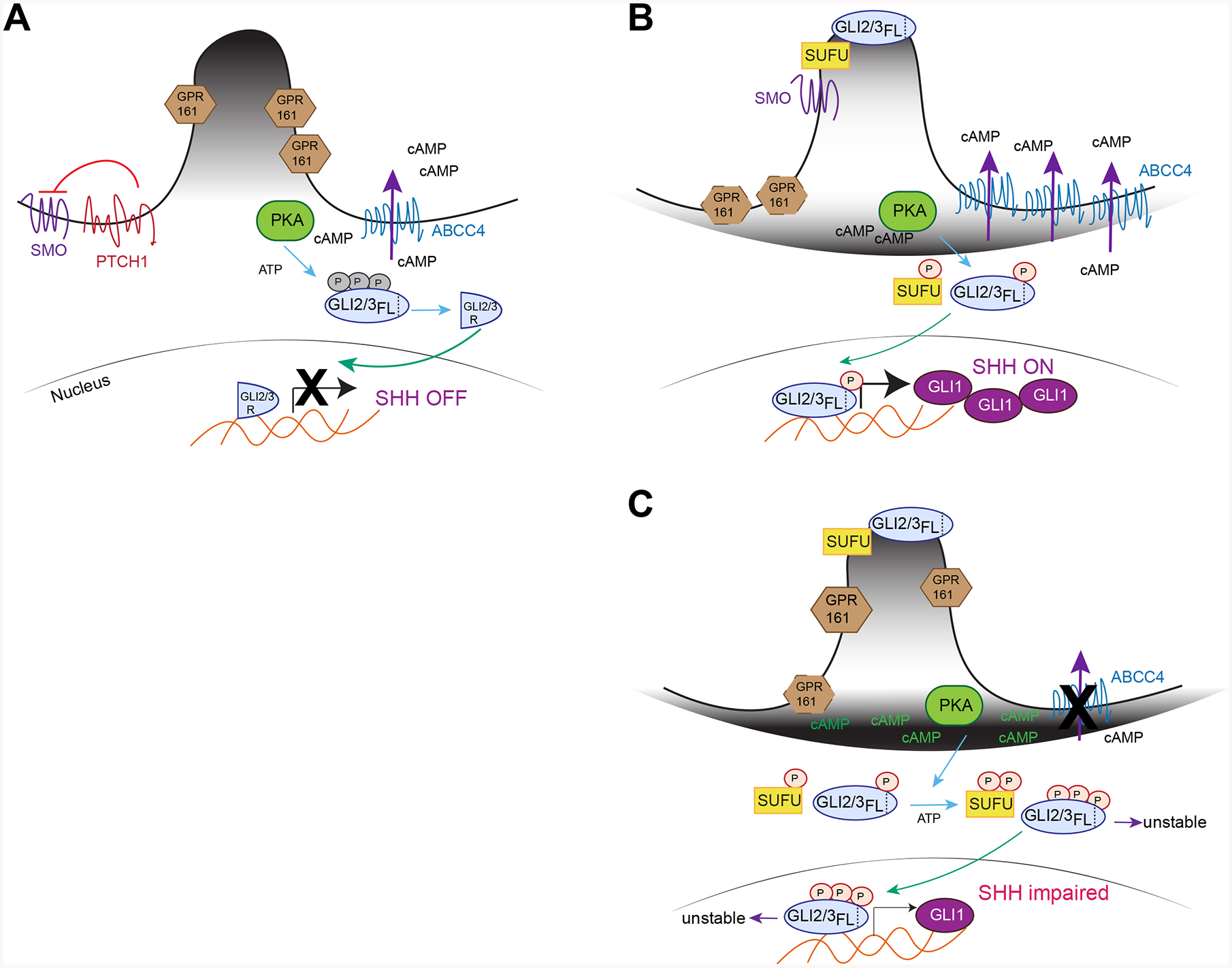
(A) In the absence of SHH agonist, PTCH prevents SMO activation and GPR161 is retained at primary cilia where it regulates cAMP level. GLI2/3 is phosphorylated by PKA at inhibitory sites and proteolytically cleaved to act as a transcriptional repressor. ABCC4 is a plasma membrane, ATP-dependent cAMP efflux transporter.
(B) When SHH binds to PTCH, SMO is activated where it enters primary cilia and induces GPR161 exit. SUFU and GLI2/3 also translocate to primary cilia, a step necessary for GLI2/3 conversion to transcriptional activator. It is unknown whether PKA activating phosphorylation on GLI2/3 occurs prior or after ciliary enrichment. When SHH pathway is active, ABCC4 is upregulated where it regulates membrane cAMP pool and PKA activity. ABCC4 acts as a restrain to allow for just enough PKA to be active to phosphorylate key SHH proteins such as GLI2/3 at specific sites required to ensure proper propagation of signaling. These PKA sites are distinct from that of (A).
(C) In the absence of ABCC4, cAMP accumulates at the plasma membrane, GPR161 ciliary exit is delayed and PKA becomes over-activated. These results in GLI3-FL destabilization, leading the reduced GLI1 level. Over-activated PKA can also alter the phosphorylation codes in GLI2/3 and SUFU that are distinct from (B).
