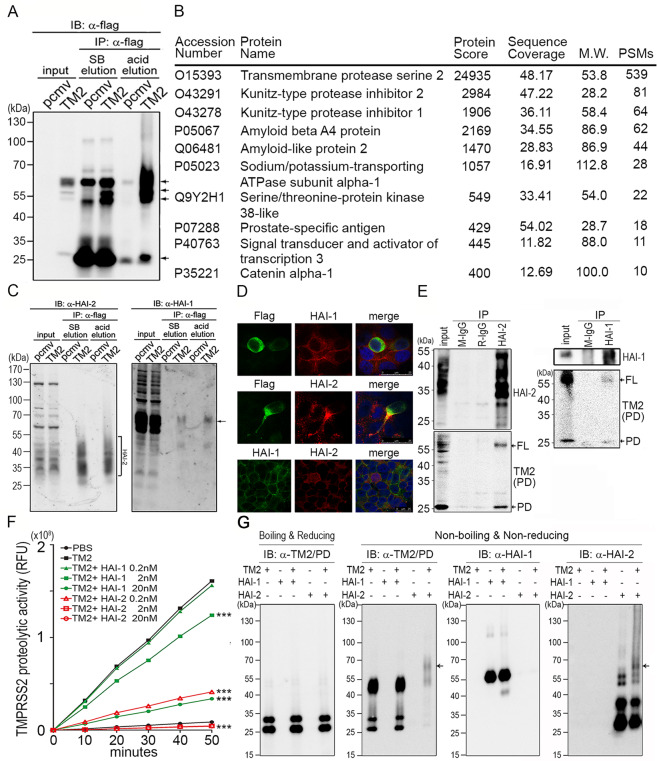
Fig. 2. Identification and validation of TMPRSS2-interacting proteins.
a Identification of TMPRSS2-interacting protein(s) by co-immunoprecipitation using M2 beads carrying anti-flag Abs. Arrows indicate the signals of TMPRSS2 or TMPRSS2 complexes. TMPRSS2-overexpressing LNCaP cells were lysed and the cell lysates were incubated with M2 beads at 4 °C, gentle rotation, overnight. The beads were then washed using TBST, four times and subjected to Laemmli sample buffer (SB) elution and acid elution, respectively. b List of proteins identified after Co-IP and LC/MS/MS analysis. The eluent from acid elution was subjected to LC/MS/MS analysis. The top ten protein identities with high #PSMs were listed in the table. c Interactions between TMPRSS2 and HAI-1/HAI-2 were validated by co-immunoprecipitation and immunoblot analysis in TMPRSS2-overexpressing LNCaP cells. The eluted proteins from immunoprecipitation were subjected to immunoblot analysis with anti-HAI-1 (Genetex) and anti- HAI-2 (LTK) antibodies. Square bracket, HAI-2. Arrows, HAI-1. d Analysis of the subcellular localization of HAI-1, HAI-2 and TMPRSS2 in PCa cells. Immunofluorescence staining of HAI-1, HAI-2 and TMPRSS2 was performed in PCa CWR22Rv1 cells. Cells were transiently transfected with TMPRSS2 plasmids using lipofectamine 3000 (Thermo, IL, USA). Two days after transfection, cells were fixed with 4% paraformaldehyde and blocked with 5% BSA plus 2% normal goat serum. Cells were stained with a monoclonal anti-Flag antibody (TMPRSS2, green), HAI-1 (M19, red) and HAI-2 (DC16, red). e Interaction between endogenous HAI-2 and TMPRSS2 in PCa cells. The interaction between HAI-2/HAI-1 and TMPRSS2 was examined in LNCaP cells using co-immunoprecipitation and immunoblot analysis. LNCaP cells were lysed and the cell lysates were incubated with an anti-HAI-2 antibody or an anti-HAI-1 antibody at 4 °C overnight. Protein G Sepharose beads were then added into the solution to isolate antibody-captured protein complexes. Normal mouse IgG (M-IgG) and rabbit IgG (R-IgG) were used as negative controls. The eluted proteins were subjected to immunoblot analysis with anti-HAI-2, anti-HAI-1 and anti-TMPRSS2 (AL-20) antibodies. FL full length of TMPRSS2. PD protease domain of TMPRSS2. f Analysis of the effects of the recombinant extracellular regions of HAI-1 and HAI-2 proteins (R&D) on the proteolytic activity of recombinant TMPRSS2 proteins by the measurement of the fluorescence signal of Boc-Gln-Ala-Arg-AMC substrate (ENZO). 0.2 nM of rTMPRSS2 proteins were incubated with the indicated concentrations of recombinant HAI-1 or HAI-2 proteins and then subjected to fluorescence detection in various time points. Measurements were performed in triplicate. Three independent experiments were performed, statistically calculated and presented as mean ± S.D. g TMPRSS2 formed complexes with HAI-2. Fifty nanograms (50 ng) of human rTMPRSS2 proteins were incubated with 50 ng of human rHAI-1 or rHAI-2 for 1 hour in PBS at 37 °C. The complex formation between rTMPRSS2 and rHAI-1 or rHAI-2 was analyzed using immunoblot analyses under boiling/reducing or non-boiling/non-reducing condition, using anti-TMPRSS2 (Abcam), anti-HAI-1(Genetex), as well as anti-HAI-2 (LTK) antibodies. The arrows indicated the complexes of rTMPRSS2 and rHAI-2 proteins.