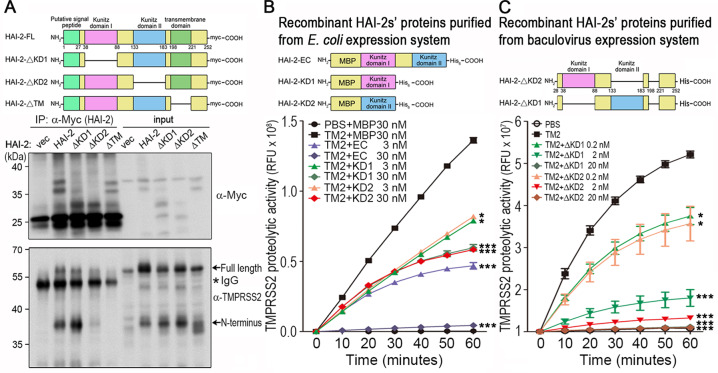
Fig. 3. Identification of the functional domain of HAI-2 for the inhibition of TMPRSS2 proteolytic activity.
a Schematic structure of wild-type and various mutants of HAI-2. Wild-type HAI-2 (HAI-2-FL), ΔKD1 HAI-2 (the deletion of kunitz domain 1), ΔKD2 HAI-2 (the deletion of kunitz domain 2) and ΔTM HAI-2 (the deletion of transmembrane domain) were constructed in pcDNA3.1 vectors with a Myc/His tag. Interaction between various HAI-2 mutants and TMPRSS2 was analyzed by co-immunoprecipitation and immunoblot analysis. HEK293 cells were transfected with wild-type HAI-2, ΔKD1 HAI-2, ΔKD2 HAI-2 and ΔTM HAI-2 (Myc tag) plasmids in the presence of TMPRSS2 (a flag tag) plasmids. Cell lysates were used for immunoprecipitation using an anti-Myc antibody. The eluted proteins were subjected to SDS-PAGE and western blotting analysis with anti-Myc or anti TMPRSS2 (Abcam) antibodies. The arrows indicated the full length or amino (N) terminus of TMPRSS2. The (*) indicated IgG. b The extracellular region (EC, a.a.38–a.a.183), kunitz domain I (KD1, a.a.38–a.a.88) and kunitz domain II (KD2, a.a.133–a.a.183) portions of HAI-2 were constructed into pMAL-c2x vectors. The plasmids were used to transform E. coli strain BL21-DE3 for the protein overexpression and purification. The effects of purified rHAI-2-EC, rHAI-2-KD1 and rHAI-2-KD2 proteins on TMPRSS2’s proteolytic activity were examined by measuring the fluorescence signals of Boc-Gln-Ala-Arg-AMC substrates. Measurements were performed in triplicate in each experiment. Three independent experiments were carried out and statistically calculated as mean ± S.D. c The HAI-2 mutants with deletion of KD1 (HAI-2-ΔKD1) or deletion of KD2 (HAI-2-ΔKD2) were constructed into Bacmid vectors for their overexpression in insect cells. The effects of purified recombinant HAI-2-ΔKD1 and HAI-2-ΔKD2 proteins on TMPRSS2’s proteolytic activity were performed by monitoring the fluorescence signals of Boc-Gln-Ala-Arg-AMC substrate. Measurements were performed in triplicate each experiment. Three independent experiments were carried out and statistically calculated as mean ± S.D.