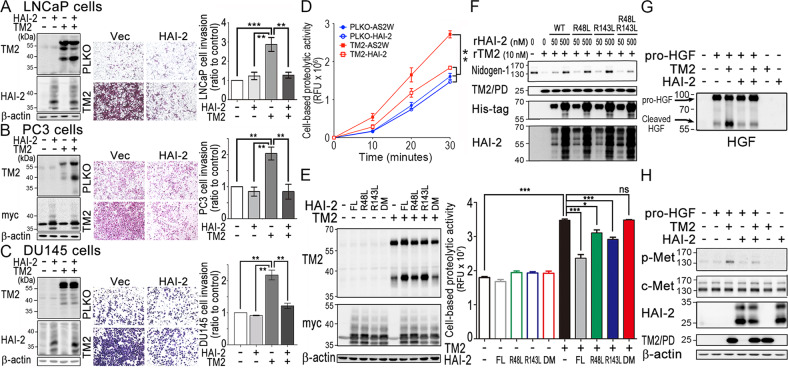
Fig. 4. Inhibitory role of HAI-2 in TMPRSS2-induced cell invasion and substrate cleavage.
a–c Role of HAI-2 in TMPRSS2-induced PCa cell invasion. PCa LNCaP (a), PC3 (b), and DU145 cells (c) were transfected with TMPRSS2 plasmids in the presence or absence of HAI-2 plasmids and stable pools were established after antibiotic selections. Control cells were transfected with pLKO (vector for TMPRSS2) or pcDNA 3.1 (vector for HAI-2). Cell lysates were collected, subjected to SDS-PAGE and immunoblot analysis using anti-TMPRSS2 (AL20) and anti-HAI-2 antibodies. β-actin was used as control. Left panels: Immunoblots of TMPRSS2 and HAI-2 in the transfectants. Middle and Right panels: Effect of TMPRSS2- or HAI-2-overexpression on the invasion of the PCa cells. For invasion assays, cells were seeded at a density of 4 × 105 (LNCaP) or 5 × 104 (PC3 and DU145) per Boyden chamber with Matrigel-coating and cultured for 40 h (LNCaP) or 16 h (PC3 and DU145). Cells were fixed and stained with crystal violet. Invaded cells were imaged under a light microscope. The cell invasion results were statistically calculated from three independent experiments and represented as mean ± SEM. d Analysis of the role of HAI-2 in TMPRSS2-induced pericellular proteolysis of PCa cells by monitoring the fluorescence signals of Boc-Gln-Ala-Arg-AMC substrate. DU145 transfectants with the overexpression of HAI-2, TMPRSS2 and TMPRSS2/HAI-2 were seeded, cultured one day, and starved for 16 h. Cells were then washed with PBS and incubated with 10 μM of fluorescence substrates. The conditioned media were then collected at indicated times and used for the measurement of the fluorescence signals. Results were statistically calculated from three independent experiments. e Analysis of the pericellular proteolytic activity of CWR22Rv1 cells with or without TMPRSS2 overexpression in the presence or absence of various HAI-2 mutants. CWR22Rv1 cells were transfected with wild-type HAI-2, HAI-2-R48L, HAI-2-R143L, HAI-2-R48L/R143L double mutant plasmids in the presence of TMPRSS2 plasmid or vector alone. Cell lysates were used for SDS-PAGE and immunoblotting analysis using anti-TMPRSS2 (AL20) or anti-Myc antibodies (Santa Cruz). β-actin was used as control. Immunoblots were shown in the left panel. The effects of TMPRSS2 and HAI-2 mutant overexpression on the pericellular proteolytic activity of CWR22Rv1 cells were performed by monitoring the fluorescence signal of Boc-Gln-Ala-Arg-AMC substrate (ENZO). For the pericellular proteolysis assays, transfectants were washed with PBS and incubated with 30 μM of fluorescence substrates. The conditioned media were then collected after incubation at 37 °C for 40 min and used for the measurement of fluorescence signals. f Examination of the effects of purified rHAI-2 mutant proteins (from E. coli) on the proteolytic activity of TMPRSS2 on Nidogen-1 cleavage. Three micrograms (3 μg) of Matrigel (Corning) were incubated with rTMPRSS2 proteins (10 nM) in the presence of different concentrations of purified recombinant HAI-2 mutant proteins with MBP/His tag. After incubation, the mixture was then subjected to SDS-PAGE and western blot analysis with anti-Nidogen-1 (GeneTex), anti-TMPRSS2 (TM2; Abcam), anti-His (Thermo) and anti-HAI-2 (LTK) antibodies. g, h Inhibitory effect of HAI-2 on TMPRSS2-induced pro-HGF cleavage and c-Met activation in PCa cells. Fifty nanograms (50 ng) of pro-HGF were incubated with 20 nM rTMPRSS2 or 200 nM rHAI-2 (R&D) in PBS for an hour, and some of the solutions after the proteolytic processing were subjected to the immunoblot analysis using an anti-HGF antibody (g). The remaining of the TMPRSS2-processing solutions was added to serum-starved DU145 cells for 10 min, and cell lysates were collected, followed by SDS-PAGE and immunoblot analysis using anti-HAI-2 (LTK), anti-TMPRS22 (TM2; Abcam), anti-phospho-Met (p-met, Cell Signaling), anti-c-Met (Spring), and anti-β-actin antibodies (h).