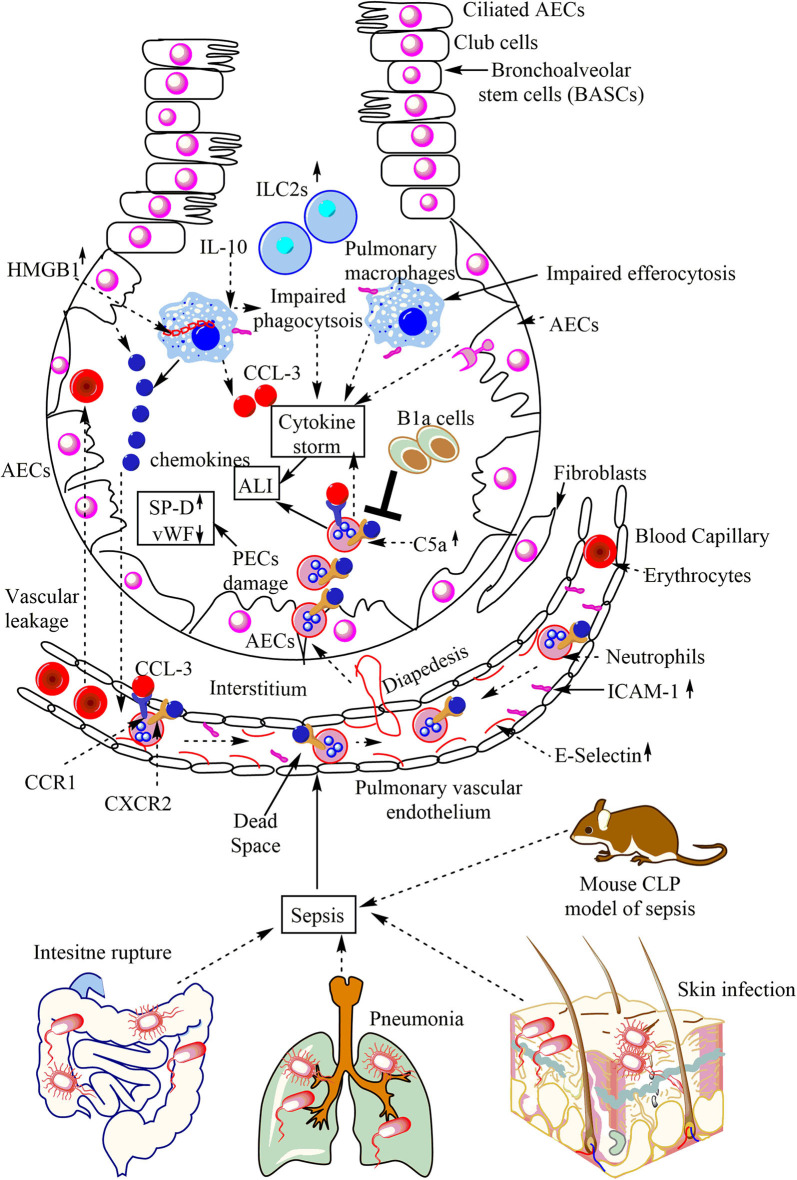
Figure 3.
Overview of sepsis-induced ALI. Local infections of the skin (S. aureus), lungs (pneumonia), and intestinal commensal bacteria leak into the blood may lead to sepsis development. Sepsis leads to the neutrophil and monocyte infiltration in the lung alveoli via pulmonary transendothelial migration due to the profound release of the pro-inflammatory mediators (cytokine storm) damaging endothelial monolayer and inducing endothelial vascular leakage. These neutrophils and monocytes reach into the lung alveoli through crossing the pulmonary epithelial layer due to damage of PECs (AECs and BECs). These PECs express C5aR and C3aR receptors. The profound release of complement component C5a during sepsis induces the inflammatory damage, and the death of PECs during sepsis causes sepsis-associated ALI. The PECs death/damage increases the SP-D, but Vwf levels decrease. The increase in the IL-10 levels in the lungs at later stages of sepsis impairs the bactericidal action of AMs along with inducing a defective efferocytosis. The defective efferocytosis among AMs further increases the ALI. The necrotic death of AMs (indicated by the cytosolic HMG-B1) at later stages of sepsis further aggravates the ALI. The neutrophils infiltrated into the lung alveoli during sepsis are apoptosis-resistant and aggravate the ALI due to their increased pro-inflammatory action on lung tissues. B1a cells inhibit neutrophil infiltration and, thus, the sepsis-induced ALI. The increase in pulmonary ILC2s also occurs.