Abstract
Background
Heart failure (HF) is a leading cause of morbidity and mortality and although it is linked to sleep apnea, which physiological stressors most strongly associate with incident disease is unclear. We tested whether sleep apnea-specific hypoxic burden (SASHB) predicts incident HF in two independent cohort studies.
Research Question
In comparison with apnea-hypopnea index (AHI), how does sleep apnea-specific hypoxic burden predict incident HF?
Study Design and Methods
The samples were derived from two cohort studies: The Sleep Heart Health Study (SHHS), which included 4,881 middle-aged and older adults (54.4% women), age 63.6 ± 11.1 years; and the Outcomes of Sleep Disorders in Older Men (MrOS), which included 2,653 men, age 76.2 ± 5.4 years. We computed SASHB as the sleep apnea-specific area under the desaturation curve from pre-event baseline. We used Cox models for incident HF to estimate the adjusted hazard ratios (HRs) for natural log-transformed SASHB and AHI adjusting for multiple confounders.
Results
The SASHB predicted incident HF in men in both cohorts, whereas AHI did not. Men in SHHS and MrOS had adjusted HRs (per 1SD increase in SASHB) of 1.18 (95% CI, 1.02-1.37) and 1.22 (95% CI, 1.02-1.45), respectively. Associations with SASHB were observed in men with both low and high AHI levels. Associations were not significant in women.
Interpretation
In men, the hypoxic burden of sleep apnea was associated with incident HF after accounting for demographic factors, smoking, and co-morbidities. The findings Suggest that quantification of an easily measured index of sleep apnea-related hypoxias may be useful for identifying individuals at risk for heart disease, while also suggesting targets for intervention.
Key Words: apnea-hypopnea index, heart failure, polysomnography, sleep apnea, sleep apnea-specific hypoxic burden
Abbreviations: AHI, apnea hypopnea index; CHD, coronary heart disease; CSA, central sleep apnea; HF, heart failure; HR, hazard ratio; MrOS, the Outcomes of Sleep Disorders in Older Men; PSG, polysomnography; SASHB, sleep apnea specific hypoxic burden; SHHS, the Sleep Heart Health Study; SpO2, oxyhemoglobin saturation
OSA is a common chronic disorder with an estimated prevalence rate of 17% in women and 22% in men,1 with prevalence exceeding 30% in older individuals.2 OSA is characterized by frequent partial or complete obstructions of the upper airway during sleep,3 leading to sleep fragmentation4 and sympathetic activation.5 The adverse consequences of untreated OSA are sleepiness and neurocognitive deficits,6, 7, 8 hypertension, cardiovascular and cerebrovascular disease, and metabolic dysfunction.9, 10, 11 Additionally, a high prevalence of OSA has been reported in patients with heart failure (HF).12, 13, 14 HF is a major cause of morbidity and mortality, resulting in over $32 billion per year of health-care costs in the United States.15,16 OSA has been implicated in increased risk for the development of HF17, 18, 19; however, there are inconsistent data, with questions on the directionality.17,18,20
In research and clinical settings, OSA is primarily characterized by the apnea-hypopnea index (AHI), which is the frequency of partial (hypopnea) and complete (apnea) airway obstructions during sleep. Using this metric, the relationship between OSA and HF has been inconsistent.17, 18, 19 For instance, although in the Sleep Heart Health Study (SHHS) a significant association between AHI and HF was observed in men,17 no such relationship was found in women or in older male participants in the Osteoporotic Fractures in Men Study (MrOS).18 In fact, data from this study showed an association between central but not obstructive apneas with incident HF.18 One interpretation is that the AHI does not accurately capture the disease burden presented by OSA and, as such, does not reflect the factors that adversely affect the cardiovascular system, ultimately leading to an increased risk of HF (eg, frequent overnight hypoxemia and increased catecholamines caused by fragmented sleep18).
Therefore, one explanation for the inconsistent data on the relationship between OSA and incident HF may be the inadequate characterization of OSA severity using frequency-based metrics (eg, AHI) or nonspecific metrics (eg, percent time with oxyhemoglobin saturation [SpO2] < 90%). Recent work from our group has demonstrated that an elevated sleep apnea-specific hypoxic burden (SASHB) directly attributable to apneas and hypopneas, calculated as the oxygen desaturation “area under the curve” in association with individual apneas and hypopneas, confers an increased risk of cardiovascular mortality in models that adjust for AHI and several other confounders in the MrOS and SHHS cohort studies.21 Given the importance of understanding the specific association between HF and OSA, we, therefore, sought to test the extent to which the SASHB predicts incident HF. We hypothesized that hypoxic burden would predict incident HF in the independent cohorts, specifically in the MrOS22, 23, 24, 25 and SHHS24,26,27 studies.
Methods
The SHHS
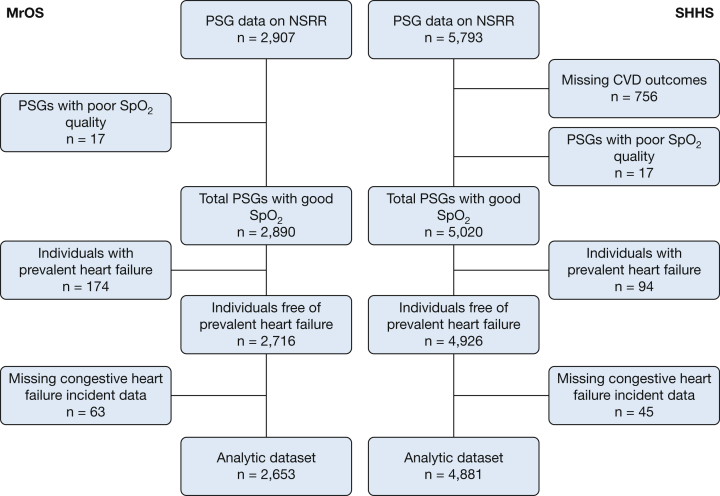
The first sample included the SHHS, a community-based, prospective cohort study designed to examine the cardiovascular outcomes of sleep-disordered breathing in middle-aged and older adults.24,26,27 This sample included 6,441 men and women aged 40 years or older who completed a baseline unattended type 2 polysomnography (PSG) (1995-1998) and a standardized questionnaire. Individuals treated with CPAP, oral devices, nocturnal supplemental oxygen, and tracheostomy were excluded.26 Of the 6,441 PSGs, 5,793 studies were available in the National Sleep Research Resource website,28 which does not include Strong Heart Study participants because of data sharing restrictions. After excluding 17 studies with missing or poor-quality SpO2 signals and all 756 from the New York University-Cornell site (outcome data not available), 94 with prevalent HF data, and 45 with missing incident HF, 4,881 individuals were available for analysis (Fig 1).
Figure 1.
Flow diagram presenting ascertainment of the study samples. CVD = cardiovascular disease; MrOS = the Outcomes of Sleep Disorders in Older Men study; NSRR = the National Sleep Research Resource; PSG = polysomnogram; SHHS = the Sleep Heart Health Study.
Consistent with prior SHHS publications,17,29 prevalent coronary heart disease was defined as a history of physician-diagnosed angina, myocardial infarction, or coronary revascularization, and it was determined by adjudicated surveillance data provided by the parent cohorts or by self-report at enrollment. Participants’ demographics and smoking history were obtained using standard questionnaires. All PSGs were scored at a central sleep reading center (Case Western Reserve University), using methods detailed before.26,27,30 Respiratory events were identified on the basis of amplitude reduction on the thermistry or respiratory inductance channels, with specific event subtypes annotated according to level of associated desaturation or EEG-based arousal. In this paper, the AHI was calculated using apneas and hypopneas both associated with desaturations of ≥ 4%, as was used in prior publications.9,17,30 SpO2 signals were captured by fingertip pulse oximetry (Nonin, Minneapolis, MN) sampled at 1 Hz.
During surveillance over a mean of 10.4 ± 3.4 years, incident HF, the primary end point for this report, was identified and confirmed using International Classification of Disease codes from hospital discharge or death certificates in the Atherosclerosis Risk in Communities Study.31 In all other cohorts, incident HF was identified by hospital and physician office records, including electrocardiograms and reports of stress tests, heart catheterizations, cardiac surgery, angioplasty, echocardiography, nuclear medicine scans, chest radiographs, and laboratory test results, using similar adjudication procedures across cohorts, as previously detailed.17,30, 31, 32, 33, 34 The criteria for HF were based on clinical symptoms, therapy, and supportive findings from chest radiographs (pulmonary edema) or cardiac imaging (eg, decreased cardiac function).17 The follow-up time was defined as the time between the baseline polysomnogram and the first HF event. The follow-up protocol followed a standardized cohort-specific protocol, as described,17 with supplemental surveillance as needed to ensure contact was made for all participants within 12 months of the final follow-up date. Multiple follow-up approaches were used, including follow-up interviews, written annual questionnaires, or telephone contacts with study participants or next-of-kin, surveillance of local hospital records and community obituaries, and linkage with the Social Security Administration Death Master File. The time from baseline evaluation to final follow-up contact was similar across parent cohorts.
MrOS
The second sample included the MrOS,35 a community-based, prospective cohort study of 5,994 men ≥ 65 years old recruited from six centers across the United States (2000-2002), designed to assess the epidemiology of osteoporosis and fractures in older men.22, 23, 24, 25 Individuals who were capable of walking without assistance and without bilateral hip replacements were eligible to participate in the study. A total of 3,135 men from the MrOS cohort participated in the ancillary MrOS sleep evaluations (from 2003 through 2005), including type 2 unattended PSG.36 Individuals on nightly use of CPAP, bilevel pressure ventilation, mouthpiece, or oxygen therapy were excluded. A total of 2,907 PSGs were available on the National Sleep Research Resource website.28 After excluding 17 studies with poor quality SpO2 signals, 174 with prevalent HF, and 63 with missing incident HF status, 2,653 participants were available for the analysis (Fig 1).
In-home sleep studies using one night of unattended PSG (Safiro, Compumedics, Inc., Melbourne, Australia) were performed as described previously and scored by the same Sleep Reading Center as for SHHS.37 Apneas were identified if thermistry airflow was absent or nearly absent for at least 10 s. Hypopneas were identified when there was at least 30% reduction in airflow (by thermistry or nasal pressure) or thoracoabdominal inductance plethysmography for at least 10 s. Similar to SHHS, the respiratory events were further annotated based on their associated desaturation or arousals. In this analysis, AHI was defined identically as described for the SHHS analysis (ie, apneas and hypopneas both associated with desaturations of ≥ 4%). SpO2 signals were captured by fingertip pulse oximeters (Nonin) sampled at 1 Hz.
Covariates and outcomes were based on measurements at the clinic examination, questionnaires, and adjudication of reported events. Participants’ demographics, medical history, and smoking status were obtained using standard questionnaires. A history of the following medical conditions was gathered: coronary heart disease, stroke, diabetes mellitus, COPD, and hypertension.
During surveillance over a mean of 8.8 ± 2.8 years after the sleep visit, participants were contacted every 4 months by postcard for potential cardiovascular events, including incident HF, with the follow-up time defined as the time from the sleep visit to the first fatal or nonfatal HF event (>97% of contacts were completed). Consistent with prior MrOS publications,18,38 incident HF was defined as hospital admission to treat increased intravascular volume (eg, pulmonary edema), low cardiac output, or both, adjudicated by a centrally trained physician after reviewing this information and the relevant hospital records, including examination of death certificates for indication of a potential cardiovascular cause.18 Proxy interviews with the next of kin were obtained when there were no medical records for out-of-hospital fatal events. For both fatal and nonfatal events, all documents were adjudicated by a board-certified cardiologist, as previously detailed.18,38
SASHB
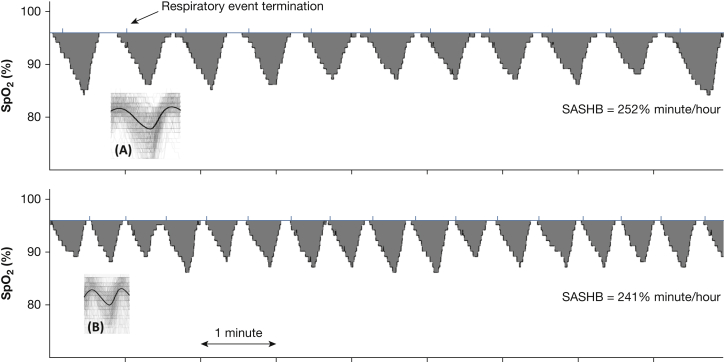
SASHB was defined as the area under the desaturation curve (below the pre-event SpO2 baseline; Fig 2) associated with respiratory events.21 By subtracting out the pre-event SpO2 baseline before calculating the area under the curve, the SASHB reflects hypoxemia caused specifically by sleep apnea, rather than by other causes of hypoxemia that would suppress the baseline SpO2. For each individually identified apnea or hypopnea (identified regardless of associated desaturation), the pre-event baseline saturation was defined as the maximum SpO2 during the 100 seconds before the end of the event. The area under this baseline value was calculated over a subject-specific search window for each event, obtained from an ensemble-averaged desaturation curve.21 Units of SASHB are %minutes/hour (a SASHB of 50 %min/h corresponds to a 5% reduction in SpO2 below baseline for 10 minutes every hour of sleep).
Figure 2.
Examples of sleep apnea-specific hypoxic burden (SASHB) for two individuals with different desaturation patterns and similar SASHBs. A and B show the overlaid oxygen saturation signals (SpO2) associated with all respiratory events for these two individuals. These signals were ensemble-averaged to obtain subject-specific search window to calculate the individual areas under desaturation curves. SASHB was the sum of individual areas divided by total sleep time. As shown, different patterns of desaturation (eg, longer, deeper, and less frequent desaturations (A) vs shorter, milder, and more frequent desaturations (B) could result in similar SASHBs.
Statistical Analysis
Distributions of covariates and sleep measures were summarized by sex and study sample. After excluding participants with HF reported before the baseline PSG, the unadjusted Kaplan-Meier cumulative incidence curves were used to qualitatively assess the association between incident HF and AHI as well as SASHB. To directly compare AHI and SASHB, we categorized both variables based on their quintiles. A series of Cox proportional models were constructed to assess the adjusted association between incident HF and SASHB (log-transformed)21 as well as incident HF and AHI (log-transformed). The proportional hazard assumption for Cox regression models was tested using the scaled Schoenfeld residuals.39 Additionally, these associations were further adjusted for competing risk of mortality using Fine and Gray models.40
Covariates were chosen based on established clinical relationships to heart failure.41,42 Furthermore, because a statistically significant interaction was observed between AHI and sex in a previous analysis of the SHHS data,17 the interaction between sex and SASHB was also added to the Cox proportional models in the SHHS. Two models were considered. Model 1, the minimally adjusted model, adjusted for age, sex (not applicable in MrOS), the interaction between sex and SASHB (or AHI), and race. Model 2 was the fully adjusted model, in which Model 1 was further adjusted for BMI, total sleep time, study site, smoking status, COPD, diabetes, hypertension, coronary heart disease (CHD, defined as history of myocardial infarction, angina, or prior coronary revascularization procedures), and stroke, with each defined as previously described.30,36 As a sensitivity analysis, model 3 assessed the association in model 2 after excluding those with a central apnea index > 5 events/hour. Also, to assess the association between SASHB and incident HF at different levels of sleepiness, an interaction term between SASHB and excessive daytime sleepiness (Epworth sleepiness score > 10) was added to model 2.
Additional sensitivity analyses were used to examine the association between incident HF and both AHI and SASHB, after excluding individuals with baseline CHD. Also, to further explore how alternative combinations of AHI and SASHB metrics influence incident HF, AHI was categorized into low (<15 events/h) and high (≥15 events/h), based on a clinical definition of moderate and severe OSA. In addition, previous studies demonstrated an increased risk of cardiovascular outcomes for AHI ≥ 15 events/hour, such that those with mild OSA had no increased risk of all-cause mortality,30 incident CHD, or HF.17 The AHI of 15 events/hour corresponded to its 75th percentile in men (SHHS and MrOS; n = 4,912). Therefore, for direct comparison, SASHB was also categorized as low (<75th percentile or SASHB = 75.2 %min/h) and high (≥75th percentile). The fully adjusted hazard ratios (HRs) of categories SASHB-Low:AHI-High (n = 205), SASHB-High:AHI-Low (n = 259), and SASHB-High:AHI-High (n = 969) were compared with the reference group, SASHB-Low:AHI-Low (n = 3,479). All statistical analyses were conducted using the R statistical package (Bell Laboratories).
Results
The characteristics of participants in MrOS and SHHS studies are shown in Table 1. Overall, 20.1% of SHHS and MrOS participants (n = 7,534) were classified as having moderate to severe OSA based on an AHI ≥ 15.
Table 1.
Sample Characteristics by Sex
| Characteristics | MrOS Men (n = 2,653) | SHHS-Men (n = 2,259) | SHHS-Women (n = 2,622) |
|---|---|---|---|
| Age, y | 76.4 (5.43) | 63.7 (10.8) | 63.6 (11.3) |
| BMI | 27.1 (3.74) | 28.5 (4.29) | 28.1 (5.63) |
| Race | |||
| White, No. (%) | 2,400 (90.5%) | 1,986 (87.9%) | 2,259 (86.2%) |
| Black, No. (%) | 91 (3.43%) | 129 (5.71%) | 191 (7.28%) |
| Other race, No. (%) | 162 (6.11%) | 144 (6.37%) | 172 (6.56%) |
| COPD, No. (%) | 129 (4.86%) | 23 (1.03%) | 30 (1.16%) |
| Smoking | |||
| Never, No. (%) | 1,065 (40.1%) | 790 (35.1%) | 1,477 (56.5%) |
| Current, No. (%) | 52 (1.96%) | 233 (10.3%) | 241 (9.22%) |
| Former, No. (%) | 1,536 (57.9%) | 1,229 (54.6%) | 897 (34.3%) |
| Diabetes, No. (%) | 333 (12.6%) | 178 (8.08%) | 159 (6.22%) |
| Hypertension, No. (%) | 1,282 (48.3%) | 891 (39.4%) | 1,010 (38.5%) |
| Follow-up time, y | 8.82 (2.81) | 10.2 (3.52) | 10.5 (3.20) |
| Prevalent CHD, No. (%) | 729 (27.5%) | 365 (16.2%) | 247 (9.44%) |
| Prevalent stroke, No. (%) | 98 (3.69%) | 68 (3.01%) | 68 (2.59%) |
| AHI, events/hour | 11.4 (12.8) | 11.0 (13.3) | 6.17 (10.1) |
| T90, % | 3.95 (9.27) | 4.58 (11.9) | 2.43 (8.30) |
| SASHB, %min/h | 57.3 (53.0) | 62.0 (64.7) | 37.0 (39.4) |
Average values are mean (SD or proportion).
AHI = apnea hypopnea index (apneas and hypopneas associated with ≥4% desaturation); CHD = prevalent coronary heart disease; MrOS = Outcomes of Sleep Disorders in Older Men study; SASHB, sleep apnea-specific hypoxic burden; SHHS = Sleep Heart Health Study; T90 = percent time below oxygen desaturation 90%.
SHHS Study
The characteristics of the SHHS participants, grouped by sex, are shown in Table 1. Although the overall Pearson correlation between AHI and SASHB in the entire sample was high (0.80), among individuals with AHI ≥ 15 and AHI ≥ 30 events/hour, the correlations were 0.66 and 0.51, respectively. This indicates that across ranges of moderate or severe levels of AHI, there is only a moderate correlation between AHI and SASHB. Mean (SD) SASHB and AHI were significantly higher in men than in women, respectively (SASHB = 62.0 (64.7) vs 37.0 (39.4) %min/h; P < .001; AHI = 11.0 (13.3) vs 6.17 (10.1) events/h; P < .001; Table 1), and the prevalence of severe OSA (measured by AHI or SASHB) was lower in women compared with men in this sample. Indeed, women only constituted 29.3% of individuals with AHI ≥ 30 events/hour (∼94th percentile of AHI) and 28.3% of individuals with SASHB ≥ 127 %minute/hour (∼94th percentile of SASHB).
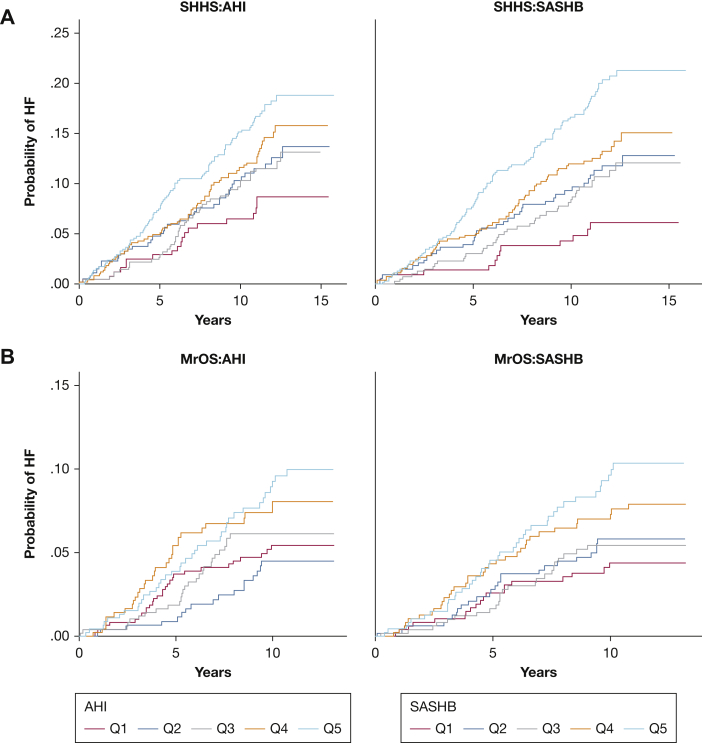
Over an average of 10.4 ± 3.4 years, there were 543 cases of incident HF (Fig 1). The rate of incident HF per 1000 person-years of follow-up was 11.9 for men and 9.2 for women, which increased with increasing AHI and SASHB (Fig 3, e-Fig 1). In SHHS, the minimally adjusted (model 1) HR of incident HF for the AHI (per 1 SD) and SASHB (per 1 SD) were 1.12 (95% CI, 1.02-1.22) and 1.14 (95% CI, 1.03-1.25), respectively. In the fully adjusted model, these associations attenuated although the point estimates were higher for SASHB (1.05 [95% CI, 0.95-1.17]) than AHI for (1.00 [95% CI, 0.91-1.11]).
Figure 3.
Unadjusted cumulative incidence Kaplan-Meier curves for quintiles of AHI (apnea-hypopnea index) and SASHB (sleep apnea-specific hypoxic burden) in men in the Sleep Heart Health Study (SHHS; A) and the Outcomes of Sleep Disorders in Older Men (MrOS; B) study.
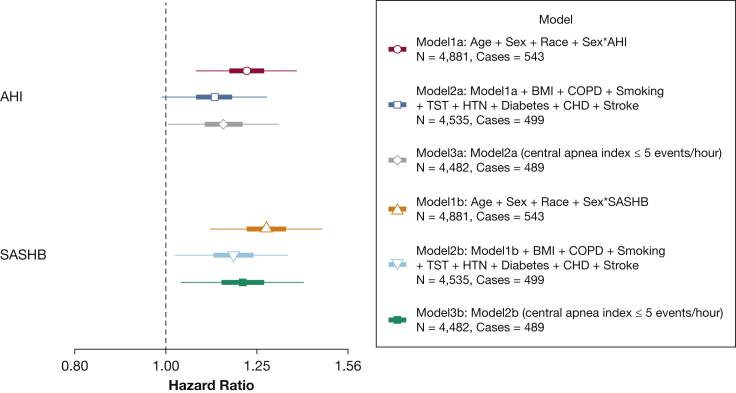
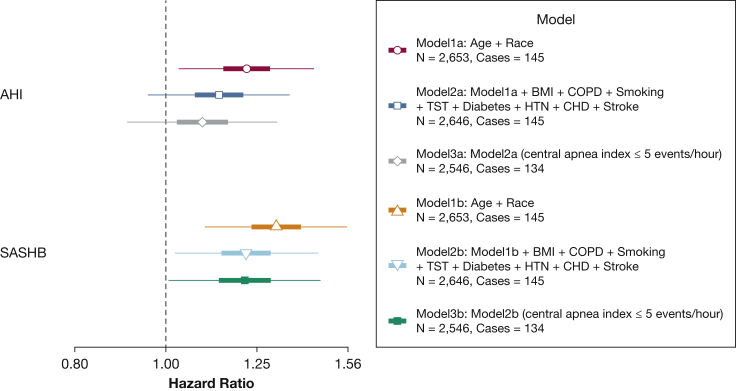
Consistent with prior analysis of associations between AHI and incident HF in SHHS, significant sex differences were evident.17 Statistically significant sex interactions were seen for both AHI (P = .044) and SASHB (P = .021). After accounting for sex interactions, the minimally adjusted HRs in men increased to 1.22 (95% CI, 1.08-1.38) and 1.28 (95% CI, 1.11-1.47) for the AHI and the SASHB, respectively (Fig 4; model 1a and model 1b). In contrast, in women, the HRs were 1.02 (95% CI, 0.89-1.15) and 1.03 (95% CI, 0.90-1.16) for the AHI and the SASHB (minimally adjusted models), demonstrating potential sex differences in the association of sleep apnea and incident HF. In the fully-adjusted model, including sex interaction and other covariates, the association of the AHI and incident HF was no longer significant in men (HR [per 1 SD], 1.12; 95% CI, 0.99-1.28; model 2a in Fig 4). In contrast, the association of the SASHB and incident HF in men persisted and remained significant (HR, 1.18; 95% CI, 1.02-1.37); model 2b in Fig 4). In women, the association of the SASHB and incident HF was not significant (HR, 0.95; 95% CI, 0.83-1.09). Excluding those with a central apnea index greater than 5 did not materially change these findings (model 3a and model 3b in Fig 4).
Figure 4.
Hazard ratio of incident heart failure (HF) in men in the Sleep Heart Health Study for 1-SD increase in the SASHB and AHI. Participants with prevalent HF were excluded. AHI and SASHB were natural log-transformed, scaled, and modeled separately. From 4,881 available participants in the analytical dataset, there were 17 missing BMI, 122 missing diabetes status, 55 missing COPD status, 14 missing smoking status, and 59 missing prevalent CHD status, resulting in 4,535 individuals in models 2a and 2b. This figure shows the effect size for men in SHHS when both men and women were included in a single model and sex interaction was added to the models. Models 3a and 3b show the effect sizes after excluding those with a central apnea index > 5 events/h. CHD = prevalent coronary heart disease including myocardial infarction, angina, and coronary revascularization. AHI = apnea-hypopnea index; HTN = hypertension; SASHB = sleep apnea-specific hypoxic burden; TST = total sleep time.
MrOS Study
Over an average of 8.8 ± 2.8 years, a total of 145 incident HF cases were identified in the MrOS cohort. The rate of incident HF per 1000 person-years of follow-up was 6.05 for men in this study, which increased with increasing AHI and SASHB (Fig 3). Similar to men in SHHS, minimally adjusted (model 1b), and fully adjusted (model 2b) Cox regression models showed a significant HR of incident HF for the SASHB (Fig 5). In the fully adjusted model, for every 1 SD increase in natural log SASHB, the risk of incident HF increased by 21.9% (HR, 1.22; 95% CI, 1.02-1.45; Fig 5). In contrast, the association between AHI and incident HF was only significant in the minimally adjusted model and became nonsignificant in the fully adjusted Cox regression model (model 2a; Fig 5). Similarly, excluding those with a central apnea index greater than 5 did not change these findings (model 3a and model 3b in Fig 5).
Figure 5.
Hazard ratio of incident heart failure (HF) in men in the Outcomes of Sleep Disorders in Older Men study for the SASHB and AHI. Participants with prevalent HF were excluded. Coronary heart disease (CHD) included myocardial infarction, angina, and coronary revascularization. AHI and SASHB were natural log-transformed and modeled separately. From 2,653 available participants in the analytical dataset, there were 2 missing BMI, 14 missing smoking status, and 6 missing prevalent CHD status, resulting in 2,646 individuals in models 2a and 2b. Models 3a and 3b show the effect sizes after excluding those with a central apnea index > 5 events/h. See Figure 4 legend for expansion of abbreviations.
Sensitivity Analyses
After excluding individuals with CHD, the fully adjusted SASHB association with incident HF strengthened in the SHHS analysis (SASHB HR in men [per 1 SD], 1.24 [95% CI, 1.04, 1.49]). A similar increase was also observed in the fully adjusted AHI model (1.18 [95% CI, 1.01, 1.38]). In MrOS, only 71 individuals had incident HF after excluding participants with baseline CHD; therefore, a similar sensitivity analysis was not conducted in the MrOS cohort.
Exploratory Analyses
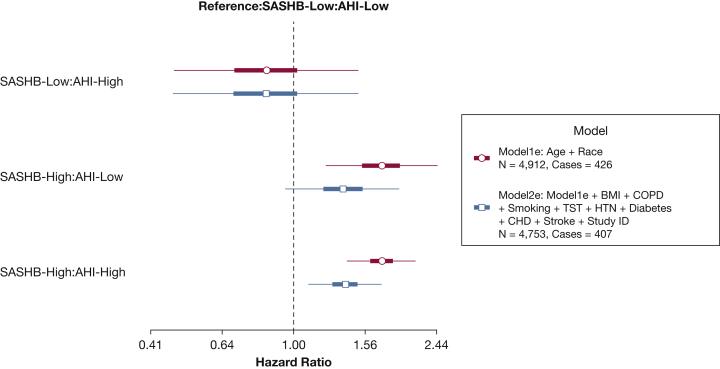
Finally, to further examine the associations of alternative combinations of exposures attributable to AHI and SASHB on incident HF, in comparison with men in the SASHB-Low:AHI-Low group (reference group, Fig 6), men with high AHI but low SASHB had an adjusted HR of 0.84 (95% CI, 0.47-1.51). In contrast, men with low AHI and high SASHB or with high AHI and high SASHB had adjusted HRs of 1.36 (95% CI, 0.95-1.95) and 1.38 (95% CI, 1.10-1.74), respectively (Fig 6), demonstrating that the depth and duration of associated desaturations may be important factors regardless of whether the AHI is less than or greater than 15. In both SHHS and MrOS cohorts, the fully adjusted associations of SASHB with incident HF in men (model 2b) remained significant after accounting for competing risk of death (SHHS: HR, 1.19 [95% CI, 1.02-1.38]; MrOS, 1.24 [95% CI, 1.03-1.50]; e-Fig 2). Finally, no significant interaction between SASHB and excessive daytime sleepiness (Epworth sleepiness score > 10) was observed (P = .65 in SHHS, P = .98 in MrOS).
Figure 6.
Hazard ratio of incident heart failure (HF) for categories of SASHB and AHI, compared with the baseline group of low SASHB and low AHI. This figure displays the pooled analysis of men in the Outcomes of Sleep Disorders in Older Men (MrOS) and the Sleep Heart Health Study (SHHS) for SASHB and AHI. Participants with prevalent HF were excluded. AHI (apnea and hypopneas associated with ≥4% desaturation) and SASHB were each categorized separately as low (<75th percentile) and high (>75th percentile), and the individuals with low AHI and low SASHB were considered the reference group. See Figure 4 legend for expansion of abbreviations.
Discussion
In this report, the SASHB was found to be associated with increased risk of incident HF in men from two large community samples of middle-aged and older adults. These associations persisted after adjusting for multiple potential confounders and possible mediators such as diabetes, hypertension, and stroke. In contrast, the association between AHI (the existing clinical metric of OSA severity) and incident HF was weaker and less precise than that observed for SASHB. Adjustment for competing risk of mortality did not change the findings. Also, excluding individuals with central apnea did not alter the findings. Therefore, these data suggest that in men, incident HF is more strongly and consistently (across cohorts) associated with a quantitative measure of sleep apnea-specific hypoxic burden than the usual measure of respiratory event frequency (AHI). Moreover, exploratory analyses indicated that an elevated SASHB index, even in the absence of an elevated AHI, is associated with incident HF.
OSA and Risk of HF
The role and directionality of the relationship between OSA and HF remain disputed.13,17, 18, 19,43,44 Using SHHS data, Gottlieb et al17 demonstrated a significant although weak association between AHI and incident HF only in men free of prevalent HF and CHD at baseline (HR, 1.13; 95% CI, 1.02, 1.26 per 10-unit increase in AHI).17 Although in the current study the association between AHI and incident HF was not significant in the fully adjusted model, a nonsignificant trend (P < .1) was observed. This inconsistency could be due to the inclusion of individuals with CHD in our analysis. Indeed, after excluding individuals with a history of CHD, our findings concerning AHI were consistent with those of Gottlieb et al,17 despite a somewhat different sample (exclusion of Strong Heart Study participants). After excluding individuals with CHD, the SASHB associations also strengthened in the SHHS analysis (SASHB HR in men [per 1 SD], 1.24 [95% CI, 1.04, 1.49]). Consistent with findings by Gottlieb et al,17 no significant interaction effect between age and AHI or SASHB on incident HF was observed. Our findings in MrOS (Fig 5) demonstrating a nonsignificant association between AHI and incident HF were also consistent with those reported by Javaheri et al.18 Therefore, the findings of the current study add to a growing recognition in the field of sleep medicine that relying on AHI and other conventional sleep parameters to capture the risk conferred by sleep apnea, including the risk of developing HF, is inadequate.19,21,45, 46, 47, 48, 49, 50 Indeed, AHI, which captures the frequency but not the “depth” of respiratory events, fails to adequately characterize the ventilatory deficit created by sleep-related upper airway obstructions. Conversely, SASHB captures both the frequency and depth aspects and thus may represent a physiologically relevant and reliably measured marker of disease burden.
Central Sleep Apnea, Hypoxemia, and Risk of HF
Prior research has shown that within HF populations, prolonged hypoxemia is predictive of mortality.19 Central sleep apnea (CSA) or Cheyne Stoke Respiration also has been shown to predict incident heart failure.18 Possibly the effects of CSA/Cheyne Stoke Respiration may be mediated through hypoxic burden. Although we were unable to calculate central apnea-pecific hypoxic burden (hypopneas were not distinguished regarding subtype), future studies with carefully annotated hypopneas may be useful for exploring this hypothesis. In the current study, we did perform a sensitivity analysis excluding those with a central apnea index > 5 events/hour, which showed little change, indicating that central apneas were not mediating effects in the overall cohort. Furthermore, too few individuals with CSA were available to address this question in this subset.
OSA-Specific Hypoxic Burden as a Predictor of Cardiovascular Outcomes
Quantifying the severity of OSA by metrics that capture the frequency of respiratory events, such as AHI and oxygen desaturation index, likely incompletely captures relevant OSA-related stresses. Indeed, a growing list of studies,19,47, 48, 49, 50 including our paper on the association between SASHB and cardiovascular-specific mortality,21 suggesting that AHI does not adequately characterize the burden of sleep apnea. SASHB was specifically designed to capture the sleep apnea-related contribution to hypoxemia, whereas metrics that do not remove the effect of baseline oxygen saturation (eg, T90, percent time with SpO2 < 90%), including the one developed by Baumert et al,47 may not relate specifically to OSA but could instead vary in relation to non-OSA exposures (such as lung disease or central hypoventilation disorders). At the same time, metrics that quantify the severity of hypoxemia tend to outperform frequency-based indexes such as AHI and ODI.19,21,47 For example, unlike AHI, T90 was shown to be an independent predictor of all-cause mortality in chronic stable HF (with reduced left ventricular function) patients, indicating the importance of hypoxemia in assessing the risk of adverse outcomes. Conversely, metrics such as T90 and other nonspecific markers,19,47 although important, may nevertheless be unrelated to the specific ventilatory deficit produced by upper airway obstruction in OSA (nonmodifiable by CPAP). In addition to metrics that quantify hypoxemia, emerging research has shown that excessively sleepy individuals are at heightened risk of incident cardiovascular disease,51 suggesting the presence of an interaction effect of OSA severity and sleepiness on cardiovascular disease. In the current study, however, no significant interaction between SASHB and excessive daytime sleepiness (Epworth sleepiness score > 10) was observed, suggesting that the association between SASHB and HF is likely independent of sleepiness; however, this needs to be independently confirmed.
Recent large trials (eg, Sleep Apnea Cardiovascular Endpoints52) have failed to find a positive long-term cardiovascular benefit of positive airway pressure treatment44,52 among individuals selected based on AHI. Our findings suggest that measures that more strongly reflect cardiovascular-related risk, such as SASHB, may be useful for selecting individuals at high risk for adverse effects of sleep apnea, and thus may respond to interventions aimed at reversing physiological stresses associated with hypoxemia.
OSA and Risk of HF in Women
Findings on the relationship between OSA and cardiovascular outcomes in men vs women have been inconsistent.17,20,53,54 Our findings of stronger associations in men compared with women are consistent with previous data reported by Gottlieb et al17 in the SHHS. However, in contrast to their findings and ours, a follow-up study of 1,645 men and women who participated in both the SHHS and the Atherosclerosis Risk in Communities study revealed a significant association between AHI and incident HF over a longer follow-up time (13.6 ± 3.2 years).54 One potential explanation for these sex-specific inconsistencies in the relationship between OSA and incident HF may be that, given the less severe exposure (quantified either by AHI or SASHB) or shorter periods of exposure (due to later onset of disease) in women (Table 1), longer follow-up time (13.6 ± 3.2 years vs 10.4 ± 3.4 in the current study) may reveal a significant association. With longer follow-up, the Atherosclerosis Risk in Communities study also demonstrated a significant association between high sensitivity troponin T, a biomarker of subclinical myocardial injury, and AHI in women.54 Therefore, we speculate that with longer follow-up time, a significant association between SASHB and incident HF may be observed, which warrants subsequent investigation. Additionally, because of lower prevalence of women with severe OSA (measured by AHI or SASHB), there was less power to detect a significant association (ie, only 29.3% of individuals with AHI ≥ 30 events/h were women).
Strengths and Limitations
There were several notable strengths of this study, including the use of an automatically generated index of SASHB, which only required the airflow and SpO2 signals. These signals are widely available in-home sleep apnea testing devices and sleep laboratories, underscoring the clinical relevance of our work. Our findings in men were robust in the face of multiple covariate adjustments, and they were consistent across two independent studies, suggesting likely generalizability. The ability to replicate findings across two independent cohorts also suggest that our findings were unlikely to be attributable to ascertainment differences or subtle differences in PSG or HF adjudication across these cohorts. However, there were several limitations, including the underrepresentation of minority populations, young individuals, and older women. In addition, because the prevalence of severe OSA, measured by AHI or SASHB, was lower in women than in men, we had less statistical power to detect a significant association in women.
In addition, another limitation was the criteria used for adjudicating incident HF events that were adopted before the 2014 ACC/AHA statement on definitions on cardiovascular events.55 For MrOS, HF was identified after surveillance of relevant medical records and death certifications and required evidence of hospitalization to treat increased intravascular volume, low cardiac output, or both. This definition would not capture cases that resulted in clinic or emergency room presentation only. The current criteria possibly could result in a more specific classification than the approaches used in this study. Nonetheless, the criteria used in each cohort were internally consistent and are unlikely to have led to a differential misclassification. Furthermore, no cardiac imaging was conducted to provide information on systolic and diastolic function, left ventricular mass and volume, and ejection fraction; therefore, there was no distinction between HF with preserved or reduced ejection fraction available for evaluation.
Despite the advantages of a single measure of SASHB that captures the depth and duration of respiratory-related desaturation, possibly alternative metrics that characterize sleep fragmentation56, 57, 58 and airflow limitation59, 60, 61, 62 also may be important in assessing outcomes such as sleepiness, cognition, and quality of life, or, combined with measures of SASHB, further improving risk stratification of cardiovascular disease.
Conclusions
The sleep apnea-specific hypoxic burden was associated with incident HF in men in two independent cohorts after considering demographic factors, smoking, sleep duration, and comorbidities. In contrast, the estimated association of AHI, the current standard for assessing sleep apnea severity, was weaker and less precise. Moreover, hypoxic burden predicted incident HF in groups with both high and low AHI levels. These findings in the current study and previous work from our group concerning mortality21 suggest that quantification of the hypoxic burden due to respiratory events may predict cardiovascular outcomes more consistently and precisely than the frequency of respiratory events.
Acknowledgments
Author contributions: A. A. was responsible for study design, analysis, interpretation of data, and drafting the manuscript. S. A. S. contributed to the analysis, interpretation of the data and critical revision of the manuscript. L. T. M., D. V., T. S., and S. W. K. contributed to interpretation of the data and critical revision of the manuscript. K. L. S. contributed to the acquisition and interpretation of the data and critical revision of the manuscript. D. P. W. contributed to the interpretation of the data and critical revision of the manuscript. A. W. contributed to the study design, analysis, interpretation of the data and critical revision of the manuscript. S. R. contributed to the study design, acquisition, analysis, interpretation of the data and critical revision of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: A. A. serves as consultant for Somnifix and Apnimed Corp. A. A. reports grant from Somnifix. S. A. S. receives personal fees as a consultant for Nox Medical and Merck outside the submitted work and receives grant support from Apnimed and Prosomnus. L. T. M. served as a consultant for Cambridge Sound Management and receives salary from Apnimed, Inc. D. P. W. receives salary from Philips Respironics and is a consultant to Apnimed, Inc. A. W. works as a consultant for Somnifix, Cambridge Sound Management, Nox Medical, Bayer, Philips, Apnimed, Inc, Inspire, and Galvani, and has received grants from Somnifix and Sanofi. A. W. and L. T. M. also have a financial interest in Apnimed, Inc, a company developing pharmacologic therapies for sleep apnea. Their interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. K. L. S. has grant funding from Merck & Co, Inc. S. R. received grant support and consulting fees from Jazz Pharmaceuticals and a consulting fee from Respicardia.
Role of sponsors: The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. A. A., A. W., and S. R. had full access to the data in the study and final responsibility for the decision to submit for publication.
Additional information: The e-Figures can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Wellman and Redline contributed equally to this manuscript. (joint last authors)
FUNDING/SUPPORT: A. A. was supported by the American Heart Association [grant 19CDA34660137] and the American Academy of Sleep Medicine Foundation [grant 188-SR-17]. S. R., S. A. S., A. W., and A. A. were partially supported by NHLBI R35HL135818. The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: The National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 TR000128, K24-AR04884-06. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. The Sleep Heart Health Study (SHHS) is supported by the National Heart, Lung, and Blood Institute through the following cooperative agreements: U01-HL53940 (University of Washington), U01-HL53941 (Boston University), U01-HL63463 (Case Western Reserve University), U01-HL53937 (Johns Hopkins University), U01-HL53938 (University of Arizona), U01-HL53916 (University of California, Davis), U01-HL53934 (University of Minnesota), U01-HL63429 (Missouri Breaks Research), and U01-HL53931 (New York University). This work was also supported by philanthropic funding from Fan Hongbing (President of OMPA Corporation, Kaifeng, China) and the National Institutes of Health [grants R01HL102321, R01HL128658, P01HL095491, UL1RR025758].
Supplementary Data
References
- 1.Franklin K.A., Lindberg E. Obstructive sleep apnea is a common disorder in the population: a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311–1322. doi: 10.3978/j.issn.2072-1439.2015.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinzer R., Vat S., Marques-Vidal P. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T., Peppard P., Gottlieb D. The epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 4.Colt H.G., Hass H., Rich G.B. Hypoxemia vs sleep fragmentation as cause of excessive daytime sleepiness in obstructive sleep apnea. Chest. 1991;100(6):1542–1548. doi: 10.1378/chest.100.6.1542. [DOI] [PubMed] [Google Scholar]
- 5.Davies C.W.H., Crosby J.H., Mullins R.L., Barbour C., Davies R.J., Sradling J.R. Case-control study of 24 hour ambulatory blood pressure in patients with obstructive sleep apnoea and normal matched control subjects. Thorax. 2000;55(9):736–740. doi: 10.1136/thorax.55.9.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White D.P., Younes M.K. Obstructive sleep apnea. Compr Physiol. 2012;2(4):2541–2594. doi: 10.1002/cphy.c110064. [DOI] [PubMed] [Google Scholar]
- 7.Engleman H.M., Kingshott R.N., Martin S.E., Douglas N.J. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS) Sleep. 2000;23(Suppl 4; Abstr):S102–S108. [PubMed] [Google Scholar]
- 8.Reynolds A.C., Banks S. Total sleep deprivation, chronic sleep restriction and sleep disruption. Prog Brain Res. 2010;185:91–103. doi: 10.1016/B978-0-444-53702-7.00006-3. [DOI] [PubMed] [Google Scholar]
- 9.Nieto F.J., Young T.B., Lind B.K. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 10.Punjabi N.M., Beamer B.A. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179(3):235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redline S., Yenokyan G., Gottlieb D.J. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Javaheri S., Parker T.J., Liming J.D. Sleep apnea in 81 ambulatory male patients with stable heart failure: types and their prevalences, consequences, and presentations. Circulation. 1998;97(21):2154–2159. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 13.Wang H., Parker J.D., Newton G.E. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49(15):1625–1631. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 14.Bitter T., Faber L., Hering D., Langer C., Horstkotte D., Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11(6):602–608. doi: 10.1093/eurjhf/hfp057. [DOI] [PubMed] [Google Scholar]
- 15.Heidenreich P.A., Trogdon J.G., Khavjou O.A. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 16.Go A.S., Mozaffarian D., Roger V.L. Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 17.Gottlieb D.J., Yenokyan G., Newman A.B. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Javaheri S., Blackwell T., Ancoli-Israel S., Ensrud K.E., Stone K.L., Redline S. Sleep-disordered breathing and incident heart failure in older men. Am J Respir Crit Care Med. 2016;193(5):561–568. doi: 10.1164/rccm.201503-0536OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oldenburg O., Wellmann B., Buchholz A. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J. 2016;37(21):1695–1703. doi: 10.1093/eurheartj/ehv624. [DOI] [PubMed] [Google Scholar]
- 20.Sin D.D., Fitzgerald F., Parker J.D., Newton G., Floras J.S., Bradley T.D. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160(4):1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 21.Azarbarzin A., Sands S.A., Stone K.L. The hypoxic burden of sleep apnoea predicted cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J. 2019;40(14):1149–1157. doi: 10.1093/eurheartj/ehy624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orwoll E., Blank J.B., Barrett-Connor E. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study: a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Blank J.B., Cawthon P.M., Carrion-Petersen M.L. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26(5):557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Dean D.A., II, Goldberger A.L., Mueller R. Scaling up scientific discovery in sleep medicine: the National Sleep Research Resource. Sleep. 2016;39(5):1151–1164. doi: 10.5665/sleep.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blackwell T., Yaffe K., Ancoli-Israel S. Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2011;59(12):2217–2225. doi: 10.1111/j.1532-5415.2011.03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan S.F., Howard B.V., Iber C. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077–1085. [PubMed] [Google Scholar]
- 27.Redline S., Sanders M.H., Lind B.K. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study: Sleep Heart Health Research Group. Sleep. 1998;21(7):759–767. [PubMed] [Google Scholar]
- 28.National sleep research resource. www.sleepdata.org
- 29.Shahar E., Whitney C.W., Redline S. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 30.Punjabi N.M., Caffo B.S., Goodwin J.L. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8) doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loehr L.R., Rosamond W.D., Chang P.P., Folsom A.R., Chambless L.E. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101(7):1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 32.The ARIC Investigators Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 33.Ives D.G., Fitzpatrick A.L., Bild D.E. Surveillance and ascertainment of cardiovascular events: the Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 34.Howard B.V., Lee E.T., Cowan L.D. Rising tide of cardiovascular disease in American Indians: the Strong Heart Study. Circulation. 1999;99(18):2389–2395. doi: 10.1161/01.cir.99.18.2389. [DOI] [PubMed] [Google Scholar]
- 35.San Francisco Coordinating Center, University of California San Francisco and California Pacific Medical Center Research Institute. MrOS online. http://mrosdata.sfcc-cpmc.net
- 36.Smagula S.F., Stone K.L., Redline S. Actigraphy- and polysomnography-measured sleep disturbances, inflammation, and mortality among older men. Psychosom Med. 2016;78(6):686–696. doi: 10.1097/PSY.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehra R., Stone K.L., Blackwell T. Prevalence and correlates of sleep-disordered breathing in older men: osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2007;55(9):1356–1364. doi: 10.1111/j.1532-5415.2007.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koo B.B., Blackwell T., Ancoli-Israel S., Stone K.L., Stefanick M.L., Redline S. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) study. Circulation. 2011;124(11):1223–1231. doi: 10.1161/CIRCULATIONAHA.111.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grambsch P.M. Goodness-of-fit and diagnostics for proportional hazards regression models. Cancer Treat Res. 1995;75:95–112. doi: 10.1007/978-1-4615-2009-2_5. [DOI] [PubMed] [Google Scholar]
- 40.Fine J., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 41.Komanduri S., Jadhao Y., Guduru S.S., Cheriyath P., Wert Y. Prevalence and risk factors of heart failure in the USA: NHANES 2013-2014 epidemiological follow-up study. J Commun Hosp Intern Med Perspect. 2017;7(1):15–20. doi: 10.1080/20009666.2016.1264696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farmakis D., Parissis J., Lekakis J., Filippatos G. Acute heart failure: epidemiology, risk factors, and prevention. Rev Esp Cardiol (Engl Ed) 2015;68(3):245–248. doi: 10.1016/j.rec.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Holt A., Bjerre J., Zareini B. Sleep apnea, the risk of developing heart failure, and potential benefits of continuous positive airway pressure (CPAP) therapy. J Am Heart Assoc. 2018;7(13) doi: 10.1161/JAHA.118.008684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowie M.R., Woehrle H., Wegscheider K. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Punjabi N.M. Counterpoint: Is the apnea-hypopnea index the best way to quantify the severity of sleep-disordered breathing? No. Chest. 2016;149(1):6–9. doi: 10.1378/chest.14-2261. [DOI] [PubMed] [Google Scholar]
- 46.Linz D., Colling S., Nussstein W. Nocturnal hypoxemic burden is associated with epicardial fat volume in patients with acute myocardial infarction. Sleep Breath. 2018;22(3):703–711. doi: 10.1007/s11325-017-1616-0. [DOI] [PubMed] [Google Scholar]
- 47.Baumert M., Immanuel S.A., Stone K.L. Composition of nocturnal hypoxaemic burden and its prognostic value for cardiovascular mortality in older community-dwelling men. Eur Heart J. 2020;41(4):533–541. doi: 10.1093/eurheartj/ehy838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muraja-Murro A., Kulkas A., Hiltunen M. The severity of individual obstruction events is related to increased mortality rate in severe obstructive sleep apnea. J Sleep Res. 2013;22(6):663–669. doi: 10.1111/jsr.12070. [DOI] [PubMed] [Google Scholar]
- 49.Zinchuk A.V., Jeon S., Koo B.B. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax. 2018;73(5):472–480. doi: 10.1136/thoraxjnl-2017-210431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butler M.P., Emch J.T., Rueschman M. Apnea-hypopnea event duration predicts mortality in men and women in the Sleep Heart Health Study. Am J Respir Crit Care Med. 2019;199(7):903–912. doi: 10.1164/rccm.201804-0758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazzotti D.R., Keenan B.T., Lim D.C., Gottlieb D.J., Kim J., Pack A.I. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med. 2019;200(4):493–506. doi: 10.1164/rccm.201808-1509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McEvoy R.D., Antic N.A., Heeley E. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 53.Young T., Finn L. Epidemiological insights into the public health burden of sleep disordered breathing: sex differences in survival among sleep clinic patients. Thorax. 1998;53(Suppl 3):S16–S19. doi: 10.1136/thx.53.2008.s16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roca G.Q., Redline S., Claggett B. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: the Atherosclerosis Risk in Communities-Sleep Heart Health Study. Circulation. 2015;132(14):1329–1337. doi: 10.1161/CIRCULATIONAHA.115.016985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hicks K.A., Tcheng J.E., Bozkurt B. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on clinical data standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards) J Am Coll Cardiol. 2015;66(4):403–469. doi: 10.1016/j.jacc.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 56.Amatoury J., Azarbarzin A., Younes M., Jordan A.S., Wellman A., Eckert D.J. Arousal intensity is a distinct pathophysiological trait in obstructive sleep apnea. Sleep. 2016;39(12):2091–2100. doi: 10.5665/sleep.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azarbarzin A., Ostrowski M., Hanly P., Younes M. Relationship between arousal intensity and heart rate response to arousal. Sleep. 2014;37(4):645–653. doi: 10.5665/sleep.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Azarbarzin A., Ostrowski M., Younes M. Arousal responses during overnight polysomnography and their reproducibility in healthy young adults. Sleep. 2015;38(8):1313–1321. doi: 10.5665/sleep.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Azarbarzin A., Moussavi Z. ICASSP; Czech Republic: 2011. Nonlinear properties of snoring sounds: the 36th International Conference on Acoustics, Speech and Signal Processing. [Google Scholar]
- 60.Azarbarzin A., Sands S.A., Marques M. Palatal prolapse as a signature of expiratory flow limitation and inspiratory palatal collapse in patients with obstructive sleep apnoea. Eur Respir J. 2018;51(2) doi: 10.1183/13993003.01419-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Camassa A., Franciosini A., Sands A. 40th Annual International Conference of the IEEE; Honololu, HI, USA: 2018. Validating an algorithm for automatic scoring of inspiratory flow limitation within a range of recording settings. Engineering in Medicine and Biology Society (EMBC); 2018. [DOI] [PubMed] [Google Scholar]
- 62.Mann D., Azarbarzin A., Mariani S. American Thoracic Society; 2018. Quantifying the severity of pharyngeal airflow obstruction using polysomnography; p. p A5935-A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.