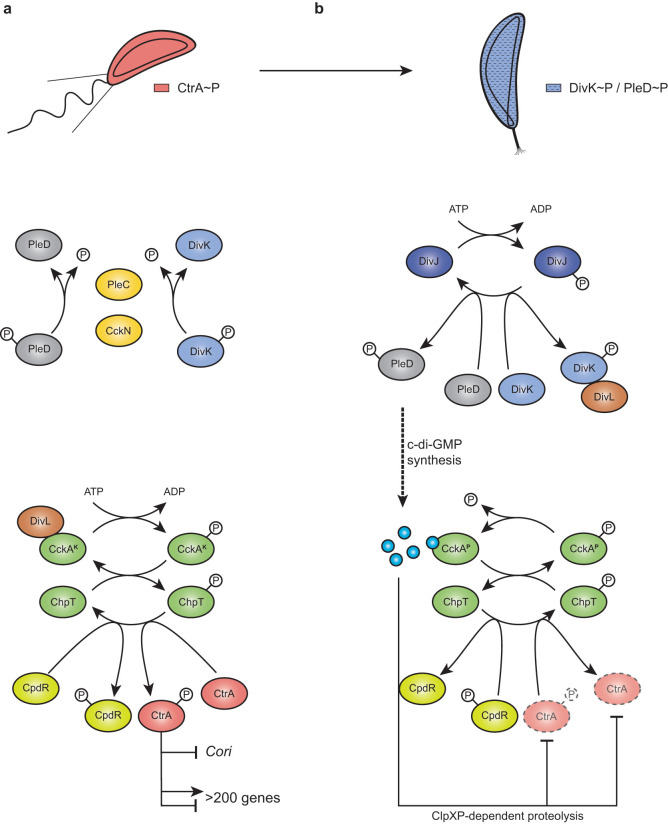
FIG 1.
(a and b) CtrA regulation pathway in Caulobacter crescentus in swarmer (a) and stalked (b) cells. In swarmer cells (a), actively dephosphorylated by PleC and CckN, DivK is therefore not able to interact with DivL. Free DivL activates the phosphorelay, culminating in CtrA and CpdR phosphorylation. Active CtrA (CtrA∼P) regulates the expression of more than 200 genes and inhibits DNA replication initiation by binding the single chromosomal origin of replication (Cori). At the G1-S transition (b), CckN and PleC are cleared from the cells, while DivK and PleD are phosphorylated by their cognate histidine kinase DivJ. Phosphorylated DivK (DivK∼P) interacts with DivL and reduces its affinity for CckA, leading to an inhibition of its kinase activity on CtrA and CpdR. Phosphorylation of PleD promotes its diguanylate cyclase activity, resulting in an increased synthesis of c-di-GMP. High levels of c-di-GMP not only stimulate CckA phosphatase activity on both CpdR∼P and CtrA∼P but also drive, concomitantly with unphosphorylated CpdR, ClpXP-dependent degradation of CtrA. Together, these effects result in the rapid inactivation of CtrA, allowing DNA replication initiation to proceed.