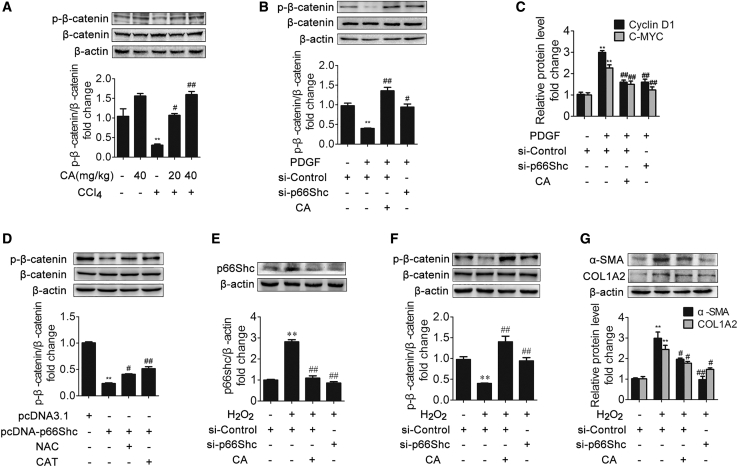
Figure 4.
p66Shc Promotes HSC Proliferation via β-Catenin Dephosphorylation
(A) Phosphorylated β-catenin and β-catenin protein expression in rat livers; n = 3. ∗∗p < 0.01 versus the control group; #p < 0.05 versus the CCl4 group; ##p < 0.01 versus the CCl4 group. (B) Phosphorylated β-catenin and β-catenin protein expression in LX-2 cells in different groups; n = 3. ∗∗p < 0.01 versus the control group; #p < 0.05 versus the PDGF-BB group; ##p < 0.01 versus the PDGF-BB group. (C) Cyclin D1 and C-MYC mRNA levels in LX-2 cells. ∗∗p < 0.01 versus the control group; ##p < 0.01 versus the PDGF-BB group. (D) Phosphorylated β-catenin and β-catenin protein expression in LX-2 cells. LX-2 cells were treated with pcDNA-p66Shc, N-acetyl cysteine (NAC), polyethylene glycol-catalase (CAT), or nothing; n = 3. ∗∗p < 0.01 versus the control group; #p < 0.05 versus the pcDNA-p66Shc group; ##p < 0.01 versus the pcDNA-p66Shc group. (E–G) p66Shc (E), phosphorylated β-catenin and β-catenin (F), and α-SMA and COL1A2 (G) protein expression in LX-2 cells from different groups. LX-2 cells were treated with CA, H2O2, SHC1 siRNA, or nothing; n = 3. ∗∗p < 0.01 versus the control group; #p < 0.05 versus the H2O2 group; ##p < 0.01 versus the H2O2 group.