Abstract
OBJECTIVE
The role of primary stereotactic radiosurgery (SRS) in patients with medically refractory acromegaly who are not operative candidates or who refuse resection is poorly understood. The aim of this multicenter, matched cohort study was to compare the outcomes of primary versus postoperative SRS for acromegaly.
METHODS
The authors reviewed an International Radiosurgery Research Foundation database of 398 patients with acromegaly who underwent SRS and categorized them into primary or postoperative cohorts. Patients in the primary SRS cohort were matched, in a 1:2 ratio, to those in the postoperative SRS cohort, and the outcomes of the 2 matched cohorts were compared.
RESULTS
The study cohort comprised 78 patients (median follow-up 66.4 months), including 26 and 52 in the matched primary and postoperative SRS cohorts, respectively. In the primary SRS cohort, the actuarial endocrine remission rates at 2 and 5 years were 20% and 42%, respectively. The Cox proportional hazards model showed that a lower pre-SRS insulin-like growth factor–1 level was predictive of initial endocrine remission (p = 0.03), whereas a lower SRS margin dose was predictive of biochemical recurrence after initial remission (p = 0.01). There were no differences in the rates of radiological tumor control (p = 0.34), initial endocrine remission (p = 0.23), biochemical recurrence after initial remission (p = 0.33), recurrence-free survival (p = 0.32), or hypopituitarism (p = 0.67) between the 2 matched cohorts.
CONCLUSIONS
Primary SRS has a reasonable benefit-to-risk profile for patients with acromegaly in whom resection is not possible, and it has similar outcomes to endocrinologically comparable patients who undergo postoperative SRS. SRS with medical therapy in the latent period can be used as an alternative to surgery in selected patients who cannot or do not wish to undergo resection.
Keywords: primary radiosurgery, stereotactic radiosurgery, acromegaly, growth hormone, pituitary adenoma, Gamma Knife, pituitary surgery
THE management of patients with acromegaly secondary to growth hormone (GH)–secreting pituitary adenomas is challenging. Persistently elevated GH levels in patients with acromegaly increases the mortality rate up to 3-fold compared to the general population.8 The cumulative cost of maintaining patients with acromegaly on lifetime medical therapy can be substantial.14 Didoni et al. found that the mean total direct costs for achieving endocrine cure for acromegaly ranged from 7968 to 12,533 euros/year.5
Stereotactic radiosurgery (SRS) is a minimally invasive therapy that offers a reasonable chance of endocrine remission for patients with acromegaly, and it does so with an acceptable risk of complications.12,15,16 In the con-temporary management of acromegaly, SRS is typically reserved for patients with residual or recurrent functioning adenoma after initial intervention with resection.6 However, primary SRS has been used as a reasonable alternative to resection in a minority of patients whose medical comorbidities unacceptably increase the risk of general anesthesia and surgery. Currently, there are few studies4,19,20 that have analyzed the results of primary SRS for acromegaly, and the effectiveness of primary versus conventional postoperative SRS for these patients is unknown. Therefore, the aim of this multicenter, retrospective matched cohort study is to compare the outcomes of primary versus postoperative SRS for the treatment of patients with acromegaly who have similar radiological and endocrinological disease burdens prior to SRS.
Methods
Patient Selection
We derived the primary and postoperative SRS cohorts from an overall database of patients with acromegaly who underwent Gamma Knife SRS between 1990 and 2016 at 7 centers participating in the International Radiosurgery Research Foundation (IRRF). The study was approved by the institutional review board of each contributing center, as well as by the IRRF review committee (protocol R-16-11). Because this was a retrospective study, patient consent was not deemed to be required. A common datasheet created at the institution of the first and senior authors was sent to the participating centers. Data from each participating center were deidentified, pooled by an independent third party, and then transmitted to the first and senior authors for analysis.
Patients with a radiological and endocrinological diagnosis of acromegaly who underwent SRS with a follow-up duration of ≥ 6 months were eligible for inclusion in the overall study cohort. Histopathological confirmation of a GH-secreting pituitary adenoma was not essential, and it was not available in the primary SRS cohort. Patients with insufficient baseline or follow-up data and those with < 6 months follow-up were excluded. Patients included in the overall study cohort were categorized into the primary versus postoperative SRS cohorts. A total of 398 patients with acromegaly comprised the original sample. From these, we identified 26 patients with primary SRS and 52 patients with postoperative SRS. The 78 patients came from the following centers: University of Virginia (19 patients), Taipei Veterans General Hospital (20 patients), University of Sherbrooke (3 patients), West Virginia University (2 patients), Charles University in Prague (19 patients), Ruber International Hospital in Spain (5 patients), and University of Pittsburgh Medical Center (10 patients).
Baseline Assessment
Baseline (i.e., pre-SRS) variables included age at SRS, sex, random GH level, insulin-like growth factor–1 (IGF-1) level, endocrinopathy, visual field defects, prior resection, time interval between most recent surgery and SRS (for those who did not have primary SRS), and type and timing of medical therapy in relation to SRS.
All patients were treated with single session of SRS performed using the Gamma Knife (Elekta AB). The Gamma Knife technique used for the treatment of pituitary adenomas has been previously described in detail.17 In brief, after placement of the stereotactic G frame (Elekta AB) on the patient’s calvaria, delineation of the target and adjacent critical structures was performed, based on high-resolution thin-slice brain MRI with contrast. A neurosurgeon, radiation oncologist, and medical physicist were involved in treatment planning. The maximal dose to the optic apparatus was generally kept below a threshold of 8 Gy.
Follow-Up Assessment
Following SRS, serial neuroimaging, comprising brain MRI with contrast, and serum hormone assessments with serum IGF-1 levels were completed at approximately 6-month intervals for the first 2 years, and then annually thereafter. Oral glucose tolerance test results were not available in many patients from afar who were unable to return for this test. Therefore, IGF-1 values were used. The endpoints for assessment were endocrine remission, radiological tumor control, adverse radiation effects (ARE), and mortality. The use of additional treatments after SRS, including medical therapy, resection, and repeat SRS were recorded.
Endocrine remission was defined as the normalization of serum IGF-1 level compared with age- and sex-matched controls while off of IGF-1–lowering medications. Tumor progression was defined radiologically as a > 20% increase compared to pre-SRS tumor size. Tumor regression was defined radiologically as a > 30% reduction compared to pre-SRS tumor size. Tumor control was defined as reduced or unchanged tumor size.18 New-onset hypopituitarism was defined as the development of a new pituitary hormone deficit after SRS.
Statistical Analysis
The normality of data was assessed with the Shapiro-Wilks test. Parametric continuous variables were reported as the mean and SD, whereas nonparametric continuous variables were analyzed using median and range. Categorical variables were reported as frequency and percentage. Patients in the primary SRS cohort were matched using
propensity scores, in a 1:2 ratio, to those in the postoperative SRS cohort based on age, sex, SRS margin dose, pre-SRS IGF-1 levels, and baseline tumor volume. The “nearest neighbor” method was used for propensity matching with a caliper of 0.20.3,13 The Mann-Whitney U-test and Student t-test were used to compare continuous variables, as appropriate. The chi-square test was used to compare categorical variables. A univariate Cox proportional hazards regression model was used to identify factors associated with initial endocrine remission and biochemical recurrence after initial remission. Covariates with p < 0.10 in the univariate analysis were entered into a multivariate model to determine independent predictors of initial remission. Kaplan-Meier analyses were performed to determine the actuarial rates of endocrine remission, tumor control, and recurrence-free survival. Actuarial rates were compared using the log-rank test. All statistical tests were 2-sided, and a p value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 25 (IBM) with R essential package for SPSS.
Results
Baseline and Treatment Characteristics
After propensity matching, the overall study cohort comprised 78 patients with acromegaly who underwent SRS, including 26 patients in the matched primary SRS cohort and 52 in the matched postoperative SRS cohort (Fig. 1). Of the 26 patients who underwent primary SRS, we obtained a response regarding the reason for this treatment approach in 25. The reason for the use of primary SRS was patient preference in 16 patients (64%), medical contraindication to surgery in 8 patients (32%), and an arterialized dural sinus in the operative corridor that pre-vented safe resection in 1 patient (4%).
FIG. 1.
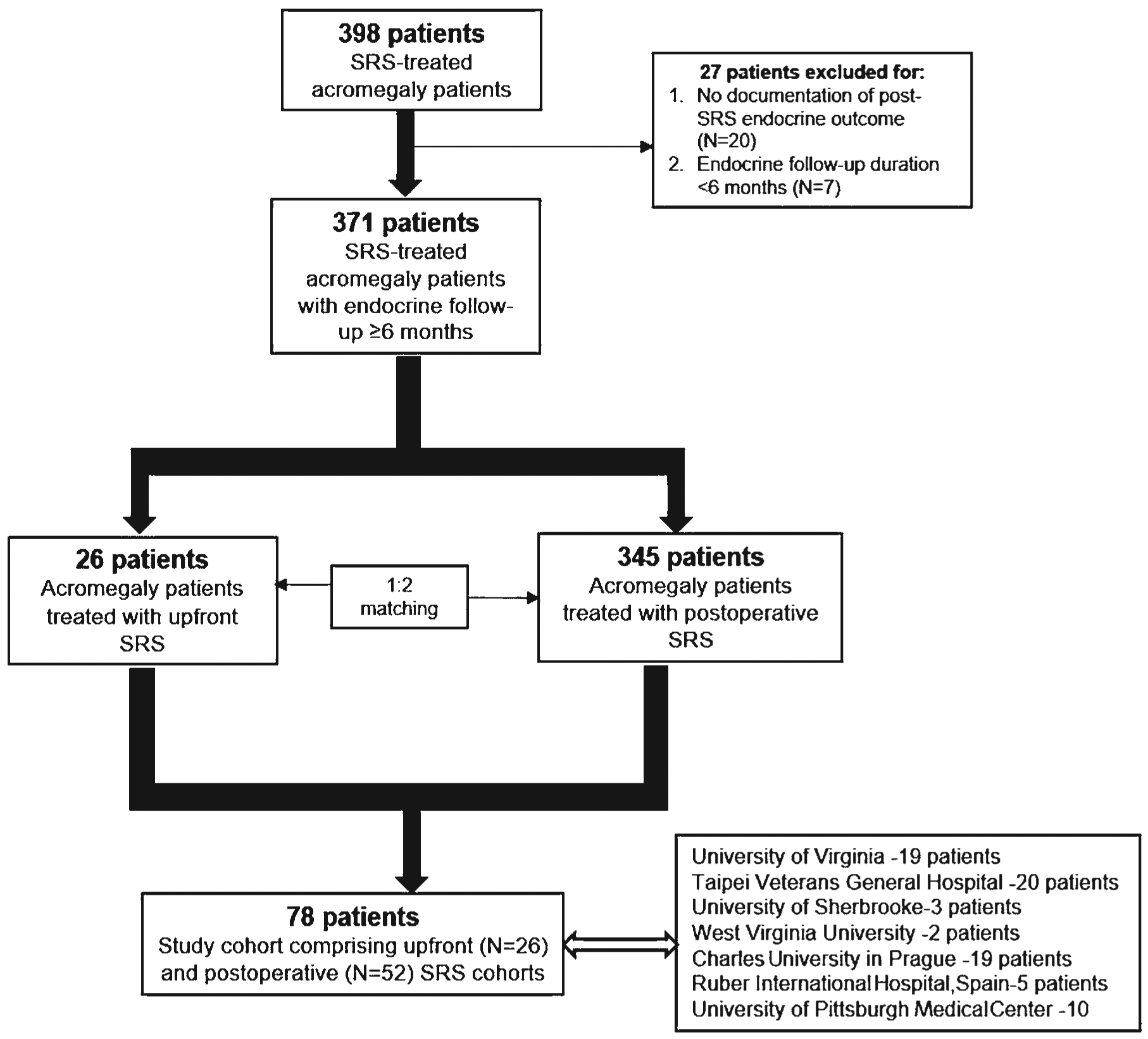
Flowchart showing the patient selection process.
Table 1 compares the baseline and treatment characteristics of the matched primary versus postoperative SRS cohorts. Each cohort was 38% male, with a median age of 58 years. The median pre-SRS serum IGF-1 levels of the matched primary and postoperative SRS cohorts were 749.5 ng/ml versus 720 ng/ml, respectively. In the matched primary SRS cohort, the median treatment volume, margin dose, and maximum dose to the optic nerves were 1.75 cm3, 23.7 Gy, and 6.6 Gy, respectively. In the postoperative SRS cohort, the median treatment volume, margin dose, and maximum dose to the optic nerves were 1.3 cm3, 25 Gy, and 5.1 Gy, respectively.
TABLE 1.
Comparison of the baseline and treatment characteristics between the matched primary and postoperative SRS cohorts
| Characteristic | Primary SRS, n = 26 | Postop SRS, n = 52 | p Value |
|---|---|---|---|
| Male sex | 10 (38%) | 20 (38%) | 1.0 |
| Median age, yrs | 58 (14–81) | 58 (17–74) | 1.0 |
| Median tumor vol, cm3 | 1.1 (0.06–7.8) | 1.0 (0.14–12.2) | 0.82 |
| Pre-SRS medical therapy | 10 (38.4%) | 15 (28.8%) | 0.39 |
| Medical therapy ongoing at time of SRS | 4 (15.3%) | 11 (21.15%) | 0.53 |
| Median pre-SRS random GH level, μg/L | 6.3 (0.5–150) | 3.4 (0.15–121) | 0.24 |
| Median pre-SRS IGF-1, ng/ml | 749.5 (65–1901) | 720 (259–1572) | 0.57 |
| Median endocrine FU duration, mos | 83.5 (11.5–235) | 60.9 (6–225) | 0.19 |
| Median radiological FU duration, mos | 66.4 (7.2–229) | 50.7 (3–182) | 0.06 |
| SRS Tx details | |||
| Median Tx vol, cm3 | 1.75 (0.14–7.8) | 1.3 (0.31–14.5) | 0.73 |
| Median margin dose, Gy | 23.7 (12–35) | 25 (12–35) | 0.25 |
| Median max dose, Gy | 44.3 (24–70) | 50 (20–70) | 0.57 |
| Median max dose to optic nerves, Gy | 6.6 (2–12) | 5.1 (0–12) | 0.07 |
| Median max dose to optic tracts, Gy | 4.2 (1.5–8.5) | 2 (0–8) | 0.04 |
| Median max dose to optic chiasm, Gy | 5.0 (2–10.0) | 3.5 (0–11.6) | 0.15 |
| Whole sella treated | 8 (30.7%) | 3 (5.7%) | 0.002 |
| Suprasellar Tx | 1 (3.8%) | 3 (5.7%) | 0.72 |
| Cavernous sinus Tx | 5 (19.2%) | 22 (42.3%) | 0.04 |
FU = follow-up; max = maximum; Tx = treatment.
All values are expressed as either the number of patients (%) or the median with range, as indicated.
The data for medical therapy were not uniformly available for all patients (53 patients did not have these data). Of the remaining patients, 25 were on pre-SRS medical therapy, 14 patients were on octreotide, and 3 patients were on lanreotide. Medical therapy was ongoing at the time of SRS in 15 patients and it was withheld just prior to SRS in 10 patients.
Endocrine Outcome
Table 2 compares the endocrine outcomes, tumor control rates, ARE, and additional treatments between matched primary and postoperative SRS cohorts. In the matched primary SRS group, the rates of initial endocrine remission, biochemical recurrence after initial remission, and durable remission were 38%, 15%, and 23%, respectively. In the matched primary SRS cohort, 10 patients (38%) achieved IGF-1 normalization off medications, and a total of 18 patients (69%) achieved normalization of IGF-1 either on or off medications. No significant differences were found between the endocrine outcomes of the 2 matched cohorts, except there was a trend toward a shorter median time to IGF-1 normalization (24.5 vs 39 months from SRS; p = 0.06) and shorter median time to biochemical recurrence after initial remission (4.4 vs 7.3 months from SRS; p = 0.06) in the matched primary SRS cohort. The actuarial rates of remission in the matched primary SRS cohort at 2 and 5 years were 20% and 42%, respectively, and these rates were similar between the 2 matched cohorts (p = 0.23; Fig. 2). The recurrence-free survival was similar between the 2 matched cohorts (p = 0.32; Fig. 3).
TABLE 2.
Comparison of the endocrine outcomes, tumor control rates, ARE, and additional treatments between the matched upfront versus postoperative SRS cohorts
| Outcomes | Primary SRS | Postop SRS | p Value |
|---|---|---|---|
| Endocrine outcome | |||
| Initial endocrine remission | 10 (38%) | 24 (46.1%) | 0.52 |
| Durable remission | 6 (23%) | 11 (21%) | 0.84 |
| Biochemical recurrence after initial remission | 4 (15%) | 13 (25%) | 0.33 |
| IGF-1 normalization on meds | 8 (30.7%) | 16 (30.7%) | 1.0 |
| IGF-1 normalization either on or off meds | 18 (69%) | 40 (76.9%) | 0.46 |
| Random GH <1 μg/L off meds | 8 (30.7%) | 23 (44.2%) | 0.25 |
| Random GH <1 μg/L on meds | 6 (23.0%) | 16 (30.7%) | 0.47 |
| Random GH <1 μg/L, either on or off meds | 14 (53.8%) | 39 (75%) | 0.06 |
| IGF-1 normalization and GH <1 μg/L off meds* | 5 (19.2%) | 19 (36.5%) | 0.12 |
| Median time to IGF-1 normalization, mos | 24.5 (10–61.6) | 39 (9–142) | 0.06 |
| Median time to recurrence after normalization of IGF-1, mos | 4.4 (2.2–16) | 7.3 (3.3–48) | 0.06 |
| Tumor control | |||
| Radiological tumor regression | 18 (69.2%) | 37 (71.1%) | 0.86 |
| Radiologically stable tumor size | 8 (30.7%) | 13 (25%) | 0.59 |
| Radiological tumor control | 26 (100%) | 50 (96.1%) | 0.32 |
| ARE | |||
| New-onset hypopituitarism | 4 (15.3%) | 10 (19.2%) | 0.67 |
| Single hormone deficiency | 2 (7.6%) | 7 (13.4%) | 0.45 |
| Multiple hormone deficiency | 2 (7.6%) | 3 (5.7%) | 0.74 |
| TSH deficiency | 2 (7.6%) | 5 (9.6%) | 0.08 |
| Testosterone deficiency | 2 (7.6%) | 4 (7.6%) | 1.0 |
| Cortisol deficiency | 1 (3.8%) | 3 (5.7%) | 0.61 |
| Cranial neuropathy excluding visual function | 0 | 0 | — |
| New visual deficits | 1 (3.8%) | 1 (1.9%) | 0.25 |
| Mortality related to Tx | 0 | 0 | — |
| Additional Txs | |||
| Resection after SRS | 1 (3.8%) | 0 | — |
| Repeat GKRS after initial SRS | 4 (15.3%) | 1 (1.9%) | 0.02 |
GKRS = Gamma Knife radiosurgery; meds = medications; TSH = thyroid-stimulating hormone; — = statistical analysis not done. All values are expressed as either the number of patients (%) or the median with range, as indicated.
2010 acromegaly consensus criteria for remission or cure.
FIG. 2.
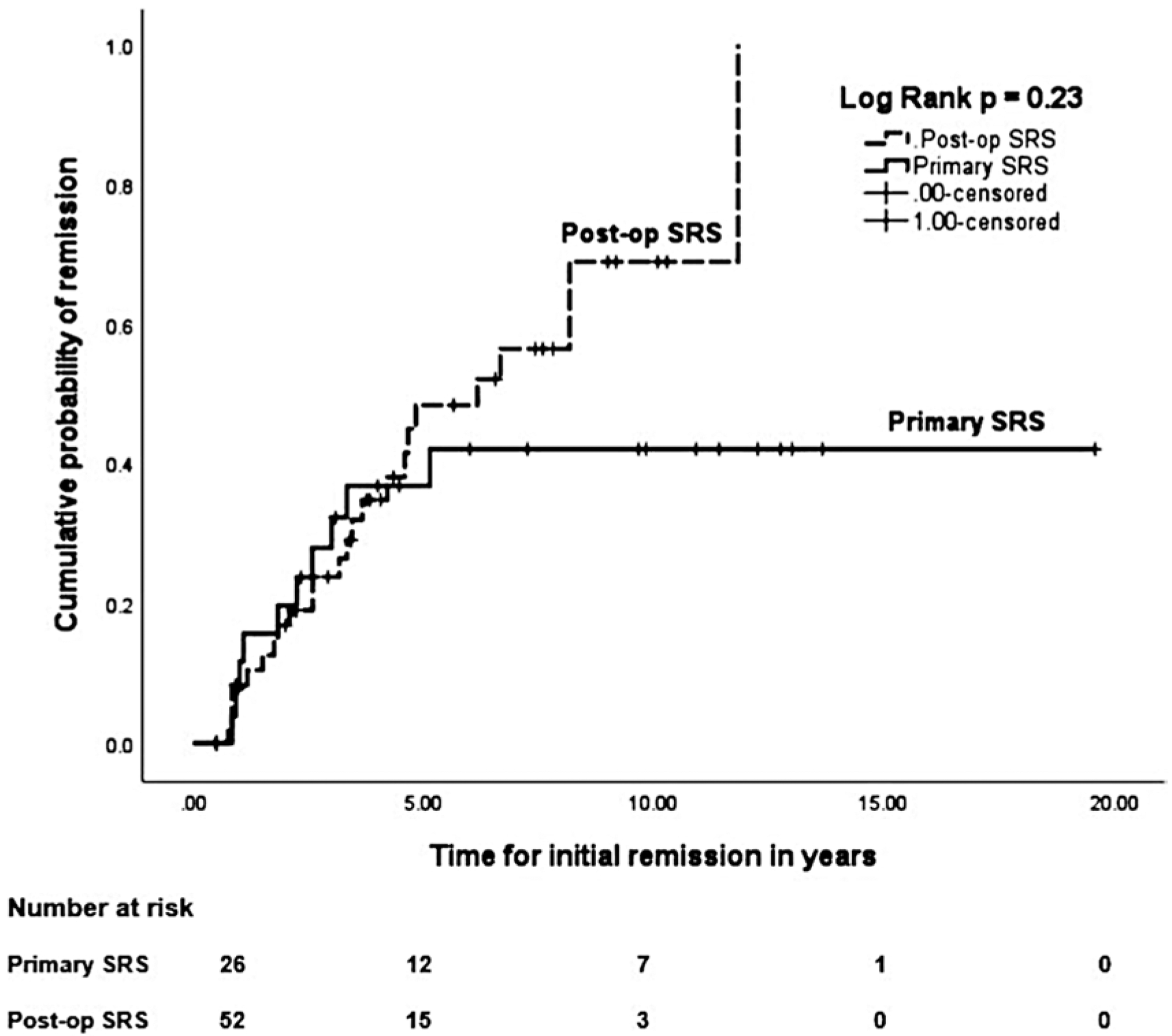
Kaplan-Meier plots comparing the actuarial initial remission rates between the matched primary versus postoperative SRS cohorts. The remission rates were similar between the 2 matched cohorts (p = 0.23, log-rank test).
FIG. 3.
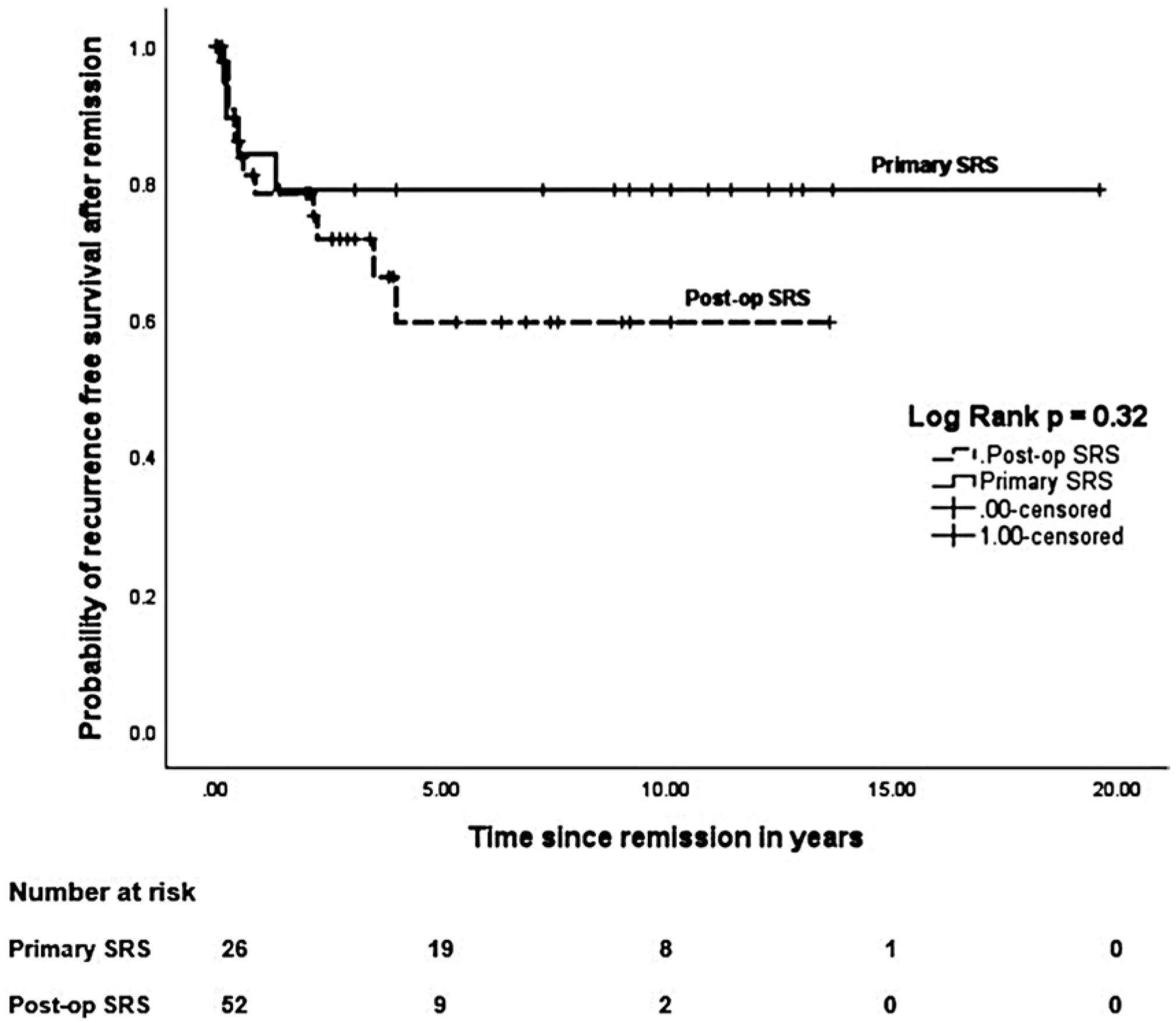
Kaplan-Meier plots comparing the actuarial rates of recurrence-free survival between the matched primary versus postoperative SRS cohorts. The recurrence-free survival rates were similar between the 2 matched cohorts (p = 0.32, log-rank test).
Radiological Outcome
In the matched primary SRS cohort, radiological tumor control was achieved in 100%, including tumor regression in 69% and stable tumor size in 31%. No significant differences were found between the radiological outcomes of 2 matched cohorts. The actuarial rates of tumor volume reduction in the matched primary SRS cohort at 2 and 5 years were 38% and 79%, respectively, and these rates were similar between the 2 matched cohorts (p = 0.34; Fig. 4). No significant differences were found between the radiological outcomes of the 2 matched cohorts.
FIG. 4.
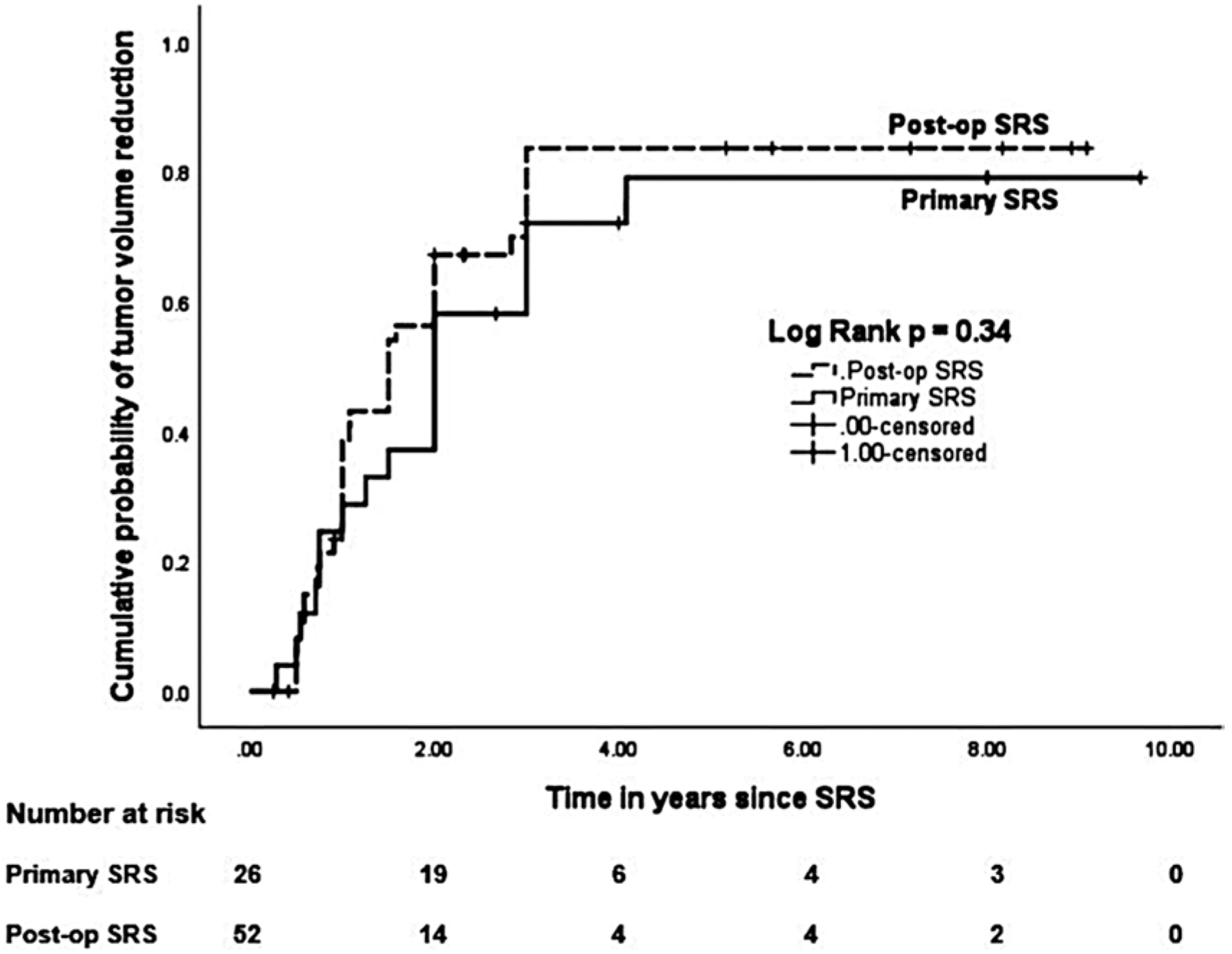
Kaplan-Meier plots comparing the actuarial tumor control rates between the matched primary versus postoperative SRS cohorts. The tumor control rates were similar between the 2 matched cohorts (p = 0.34, log-rank test).
Post-SRS Complications and Salvage Treatment
In the matched primary SRS group, a total of 4 patients (15.3%) developed new-onset hypopituitarism after SRS, including 2 with deficiency of a single hormone and 2 with deficiencies of multiple hormones. One patient in the matched primary SRS cohort developed a new visual deficit after SRS, and in this patient the maximum dose to the optic apparatus was 4.5 Gy and post-SRS tumor volume was stable.
Four patients in the matched primary SRS cohort were dead at follow-up (mortality rate 15%). The cause of death was myocardial infarction in 1 patient and unknown in the remaining 3. All but one patient among those who died were in endocrine remission off medications. Additional treatments in the matched primary SRS cohort included resection in 1 patient (4%) and repeat SRS in 4 (15%). The median time from the initial to the repeat SRS procedure was 47.5 months. None of the patients who underwent repeat SRS after primary SRS had achieved endocrine remission off medication at last follow-up. Three of these patients had achieved radiological tumor regression and 1 had stable tumor size after initial SRS. There was a trend toward increased use of repeat SRS in the matched primary SRS cohort (15.3% vs 1.9%; p = 0.02).
Predictors of Initial Endocrine Remission and Biochemical Recurrence in Patients Treated With Primary SRS
Table 3 details the univariate and multivariate Cox proportional hazards regression analyses for predictors of initial remission rate in the matched primary SRS, postoperative SRS, and combined cohorts. In the primary SRS group, pre-SRS serum IGF-1 level was the only predictive factor in either the univariate (p = 0.01) or multivariate (p = 0.03) analyses. In the univariate, a lower margin dose was found to be predictive for biochemical recurrence in the matched primary SRS group (HR 0.81, 95% CI 0.67– 0.98; p = 0.02).
TABLE 3.
Univariate and multivariate Cox proportional hazards regression analyses for predictors of initial endocrine remission in the matched primary SRS, postoperative SRS, and combined cohorts
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | p Value | HR | 95% CI | p Value | HR | 95% CI |
| Primary SRS | ||||||
| Age <60 yrs | 0.07 | 0.306 | 0.085–1.101 | 0.953 | 0.954 | 0.201–4.519 |
| Male sex | 0.34 | 0.517 | 0.133–2.00 | — | ||
| Pre-SRS serum IGF-1 level | 0.018 | 0.997 | 0.995–1.000 | 0.037 | 0.998 | 0.995–1.000 |
| Tx vol | 0.311 | 1.154 | 0.875–1.524 | — | ||
| Max dose | 0.756 | 0.994 | 0.957–1.033 | — | ||
| Margin dose | 0.350 | 0.959 | 0.879–1.047 | — | ||
| Whole sellar Tx | 0.073 | 3.157 | 0.899–11.09 | 0.119 | 3.264 | 0.737–14.45 |
| Medical therapy during SRS | 0.700 | 1.396 | 0.255–7.644 | — | ||
| Postop SRS | ||||||
| Age <60 yrs | 0.895 | 0.945 | 0.408–2.189 | |||
| Male sex | 0.899 | 1.055 | 0.460–2.419 | |||
| Pre-SRS serum IGF-1 level | 0.037 | 0.999 | 0.999–1.000 | 0.010 | 0.998 | 0.997–1.000 |
| Tx vol | 0.385 | 0.913 | 0.742–1.122 | — | ||
| Max dose | 0.102 | 1.024 | 0.995–1.053 | 0.460 | 1.017 | 0.973–1.063 |
| Margin dose | 0.503 | 1.021 | 0.961–1.086 | — | ||
| Whole sellar Tx | 0.040 | 3.660 | 1.059–12.64 | 0.010 | 5.656 | 1.519–21.06 |
| Suprasellar Tx | 0.005 | 6.306 | 1.749–22.72 | 0.001 | 10.204 | 2.483–41.93 |
| Medical therapy during SRS | 0.603 | 0.755 | 0.262–2.175 | — | ||
| Combined group | ||||||
| Age <60 yrs | 0.153 | 0.604 | 0.303–1.206 | — | ||
| Male sex | 0.824 | 0.925 | 0.467–1.834 | — | ||
| Pre-SRS serum IGF-1 level | <0.001 | 0.998 | 0.997–0.999 | <0.001 | 0.998 | 0.996–0.999 |
| Tx vol | 0.972 | 0.997 | 0.868–1.146 | — | ||
| Max dose | 0.498 | 0.986 | 0.941–1.034 | — | ||
| Margin dose | 0.571 | 1.023 | 0.983–1.065 | — | ||
| Whole sellar Tx | 0.015 | 2.728 | 1.219–6.104 | 0.003 | 3.776 | 1.573–9.068 |
| Suprasellar Tx | 0.021 | 4.212 | 1.245–14.25 | 0.012 | 5.371 | 1.451–19.88 |
| Medical therapy during SRS | 0.902 | 0.945 | 0.368–2.313 | — | ||
Boldface type indicates statistical significance.
Discussion
Although resection remains the mainstay in the management of GH-secreting pituitary adenomas, it may not be advisable or preferred by patients in all cases. The definition of endocrine remission incorporates IGF-1 level, GH level, and supplementation of IGF-1–lowering medications. Discordance between serum GH and elevated IGF-1 levels may be seen after treatment of acromegaly; this is more frequently observed after SRS.1 The acromegaly consensus group in 2010 defined optimal disease control (i.e., posttreatment remission of acromegaly) as both normalization of IGF-1 level compared to age- and sex-matched controls, and random GH level < 1.0 μg/L. In this multicenter, retrospective matched cohort study, we present the first direct comparison of primary versus postoperative SRS for patients with acromegaly who have similar endocrinological and radiological disease profiles.
Table 4 summarizes the available literature describing primary SRS for GH-secreting pituitary adenomas. Zhang et al.20 published a series of 68 patients with acromegaly who were treated with primary SRS. These investigators found normalization of GH level in 40% of the patients at 12 months. However, that study was published prior to the 2010 consensus on remission, and it considered a rather large serum GH value < 12 ng/ml as a criterion for remission.7 This probably explains the high remission rate reported in this study, and it makes comparison with recent studies difficult. A study by Thorén et al.19 reviewed 7 patients with acromegaly who underwent primary SRS. The remission criterion was a serum GH level < 2 μg/L, and 3 patients achieved remission. Castinetti et al.4 evaluated primary SRS in 19 patients with acromegaly and compared the results to 63 patients who received postoperative SRS. The 2 groups differed in age, which was higher in the primary SRS group. The investigators used the 2010 consensus on remission criteria, and after a mean follow-up of 61 months they reported endocrine remission rates of 21% and 16% in the primary versus postoperative SRS groups, respectively. The endocrine remission rate in the present study for the matched primary SRS group, in accordance with the 2010 criteria (19.2% with median follow-up of 83.5 months), is similar to that reported in the aforementioned study by Castinetti et al.
TABLE 4.
Literature review of upfront SRS for GH-secreting pituitary adenomas
| Study | No. of Pts | Margin Dose, Gy (range) | Endocrine Remission (%) | Remission Criteria | Tumor Control | Mos of Follow-Up (range) | No. w/Hypopituitarism (%) |
|---|---|---|---|---|---|---|---|
| Castinetti et al., 2005 | 19 | Mean 30 (20–35) | 21% | IGF-1 normalization w/GH < 2 ng/ml | NS | Mean 61 (19–99) | NS |
| Zhang et al., 2000 | 69 | Mean 31.3 | 96% at 24 mos | GH < 12 ng/ml | 87% at 2 yrs | Mean 34 (6–52) | 3 (4.3%); hypogonadism |
| Thorén et al., 1991 | 7 | NS | 3/7 (42.8%) | Serum GH level < 2 μg/L | 85.7% | Mean 96 (22–249) | None |
| Kim et al., 1999 | 11 | 28.7 | 45.5% | GH < 5 ng/ml | 57.1% | Mean 26.9 | None |
| Present study | 26 | Median 23.7 | 42% at 5 yrs | IGF-1 normalization off meds | 100% | Median 83.5 (11.5–235) | 4 (15.3%); hypopituitarism |
NS = not specified; Pts = patients.
In our study, the primary SRS group showed an appreciable decrease in tumor size (> 30% reduction from baseline size) in 69%, and the remaining 31% of the patients had stable tumor size. Thus, radiological control of tumor growth was achieved in all 26 patients treated with primary SRS. By comparison, tumor control rates in previous studies of primary SRS for acromegaly were 57%–87%4,10,19,20 (Table 4). Landolt et al.11 found that ongoing medical therapy during SRS could impact the effect of this intervention. Specifically, they noted that patients who were on octreotide during SRS took longer to achieve endocrine remission. Medical therapy during SRS may increase the radioresistance of tumor cells by altering the cell cycle dynamics. Therefore, discontinuation of medical therapy prior to SRS has also been incorporated as a clinical practice guideline.9 The relationship between cessation of medical therapy and improved endocrine outcomes after SRS has been suggested by a few other studies,6,15 although others have shown no association in this regard.2,4 In the present study, we hypothesized that the effect of medical therapy on the primary SRS cohort could be assessed without the confounding factor of surgery, thereby revealing the direct relationship between medical therapy and SRS. Our study did not find any significant effect of ongoing medical therapy at the time of primary SRS on endocrine outcomes. However, our primary SRS cohort suffers from low statistical power. Further studies with larger cohorts could give us a better understanding of this phenomenon.
We found a trend toward a shorter time to biochemical recurrence after initial remission (4.4 vs 7.3 months; p = 0.06) in patients who underwent primary SRS. In patients with primary SRS, the anatomical targeting of the pituitary adenoma may be more pristine as compared to that in patients who underwent SRS postresection, whose adenomas are often obscured by postoperative changes on MRI. Additionally, patients treated with primary SRS may have been more likely to undergo repeat SRS (15.3% vs 1.9%; p = 0.02). In previous studies, we have noted that the volume of the adenoma at the time of SRS was a predictor of durable endocrine remission. Thus, in general for patients who are eligible for a resection, cytoreductive surgery—even if complete resection is deemed unlikely—could afford patients with acromegaly a more durable remission after SRS. A lower pre-SRS serum IGF-1 level was the only predictor of initial remission after primary SRS, which is consistent with prior studies reporting an inverse relationship between baseline endocrinological disease severity and post-SRS outcomes.6,17 In the analysis of the combined cohort, whole sellar and suprasellar treatments were found to be independent predictors of initial remission. The concept of whole sellar treatment in primary SRS is based on the principle of addressing the entire macroscopic and microscopic disease burden in order to achieve endocrine remission.
Endocrine remission after SRS is, at times, coupled with the development of one or more new pituitary hormone deficiencies. Because both of the matched cohorts underwent SRS, our comparison assessed the effect of prior surgical decompression and a reduction in target volume on posttreatment hypopituitarism. The rates of new-onset hypopituitarism after SRS were 15% and 19% in the matched primary and postoperative SRS cohorts, respectively (p = 0.67). This could suggest that resection prior to SRS may not significantly protect against the development of hypopituitarism in this setting.
Study Limitations
The most notable limitation of this study is the modest size of the primary SRS cohort (n = 26), as well as its retrospective design, which subjects it to the selection, treatment, and referral biases of the contributing centers and their physicians. Given that the cohort size resulted in relatively low statistical power for our analyses, we may have failed to identify pertinent factors associated with endocrine outcomes after primary SRS. A greater proportion of patients in the matched postoperative SRS group had cavernous sinus treatment (42% vs 19%; p = 0.04), which reflects a greater extent of cavernous sinus invasion in this group. We did not account for this factor in the matching process, so it could have biased the comparison of post-SRS endocrine outcomes and cranial neuropathies between the 2 cohorts.
The sensitivity of the assays used to assess serum GH and IGF-1 levels may differ across the various contributing centers, thereby leading to potential discrepancies in the values obtained. This lack of assay standardization may have affected the reported endocrine results. The tumor sizes of the matched primary (median volume 1.1 cm3) and postoperative (median volume 1.0 cm3) SRS cohorts were relatively small, and therefore our findings may not be generalizable to primary SRS for medium- or large-volume GH-secreting pituitary adenomas.
Conclusions
For patients with acromegaly in whom resection is not deemed advisable or acceptable, primary SRS affords a reasonable likelihood of endocrine remission and a high rate of tumor control, and it does so with an acceptable complication rate. Primary and postoperative SRS yield similar outcomes for patients with acromegaly, with comparable endocrinological and radiological results, although patients who undergo primary SRS may have a shorter time to biochemical recurrence after initial endocrine remission and may undergo additional treatment with repeat SRS more frequently. The severity of baseline endocrinological disease burden inversely correlates with the likelihood of endocrine remission after primary SRS for acromegaly. SRS with medical therapy can be used as an alternative to surgery in selected patients who cannot or do not wish to undergo resection.
ABBREVIATIONS
- ARE
adverse radiation effects
- GH
growth hormone
- HR
hazard ratio
- IGF-1
insulin-like growth factor–1
- IRRF
International Radiosurgery Research Foundation
- SRS
stereotactic radiosurgery
Footnotes
Disclosures
Dr. Kano reports that he has an Elekta research grant. Dr. Lunsford is a shareholder in Elekta AB, and a consultant for Insightec DSMB.
References
- 1.Alexopoulou O, Bex M, Abs R, T’Sjoen G, Velkeniers B, Maiter D: Divergence between growth hormone and insulin-like growth factor-I concentrations in the follow-up of acromegaly. J Clin Endocrinol Metab 93:1324–1330, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Attanasio R, Epaminonda P, Motti E, Giugni E, Ventrella L, Cozzi R, et al. : Gamma-knife radiosurgery in acromegaly: a 4-year follow-up study. J Clin Endocrinol Metab 88:3105–3112, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Austin PC: An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46:399–424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castinetti F, Taieb D, Kuhn JM, Chanson P, Tamura M, Jaquet P, et al. : Outcome of gamma knife radiosurgery in 82 patients with acromegaly: correlation with initial hypersecretion. J Clin Endocrinol Metab 90:4483–4488, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Didoni G, Grottol S, Gasco V, Battistini M, Ferone D, Giusti M, et al. : Cost-of-illness study in acromegalic patients in Italy. J Endocrinol Invest 27:1034–1039, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Ding D, Mehta GU, Patibandla MR, Lee CC, Liscak R, Kano H, et al. : Stereotactic radiosurgery for acromegaly: an international multicenter retrospective cohort study. Neurosurgery 84:717–725, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freda PU, Post KD, Powell JS, Wardlaw SL: Evaluation of disease status with sensitive measures of growth hormone secretion in 60 postoperative patients with acromegaly. J Clin Endocrinol Metab 83:3808–3816, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Holdaway IM, Rajasoorya RC, Gamble GD: Factors influencing mortality in acromegaly. J Clin Endocrinol Metab 89:667–674, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, et al. : Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99:3933–3951, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Huh R, Chang JW, Park YG, Chung SS: Gamma Knife radiosurgery for functioning pituitary adenomas. Stereotact Funct Neurosurg 72 (Suppl 1):101–110, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Landolt AM, Haller D, Lomax N, Scheib S, Schubiger O, Siegfried J, et al. : Octreotide may act as a radioprotective agent in acromegaly. J Clin Endocrinol Metab 85:1287–1289, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Laws ER, Sheehan JP, Sheehan JM, Jagnathan J, Jane JA Jr, Oskouian R: Stereotactic radiosurgery for pituitary adenomas: a review of the literature. J Neurooncol 69:257–272, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Lunt M: Selecting an appropriate caliper can be essential for achieving good balance with propensity score matching. Am J Epidemiol 179:226–235, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore DJ, Adi Y, Connock MJ, Bayliss S: Clinical effectiveness and cost-effectiveness of pegvisomant for the treatment of acromegaly: a systematic review and economic evaluation. BMC Endocr Disord 9:20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollock BE, Jacob JT, Brown PD, Nippoldt TB: Radiosurgery of growth hormone-producing pituitary adenomas: factors associated with biochemical remission. J Neurosurg 106:833–838, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Pomeraniec IJ, Kano H, Xu Z, Nguyen B, Siddiqui ZA, Silva D, et al. : Early versus late Gamma Knife radiosurgery following transsphenoidal surgery for nonfunctioning pituitary macroadenomas: a multicenter matched-cohort study. J Neurosurg 129:648–657, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheehan JP, Pouratian N, Steiner L, Laws ER, Vance ML: Gamma Knife surgery for pituitary adenomas: factors related to radiological and endocrine outcomes. J Neurosurg 114:303–309, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Snell JW, Sheehan J, Stroila M, Steiner L: Assessment of imaging studies used with radiosurgery: a volumetric algorithm and an estimation of its error. Technical note. J Neurosurg 104:157–162, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Thorén M, Rähn T, Guo WY, Werner S: Stereotactic radiosurgery with the cobalt-60 gamma unit in the treatment of growth hormone-producing pituitary tumors. Neurosurgery 29:663–668, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Zhang N, Pan L, Wang EM, Dai JZ, Wang BJ, Cai PW: Radiosurgery for growth hormone-producing pituitary adenomas. J Neurosurg 93 (Suppl 3):6–9, 2000 [DOI] [PubMed] [Google Scholar]


