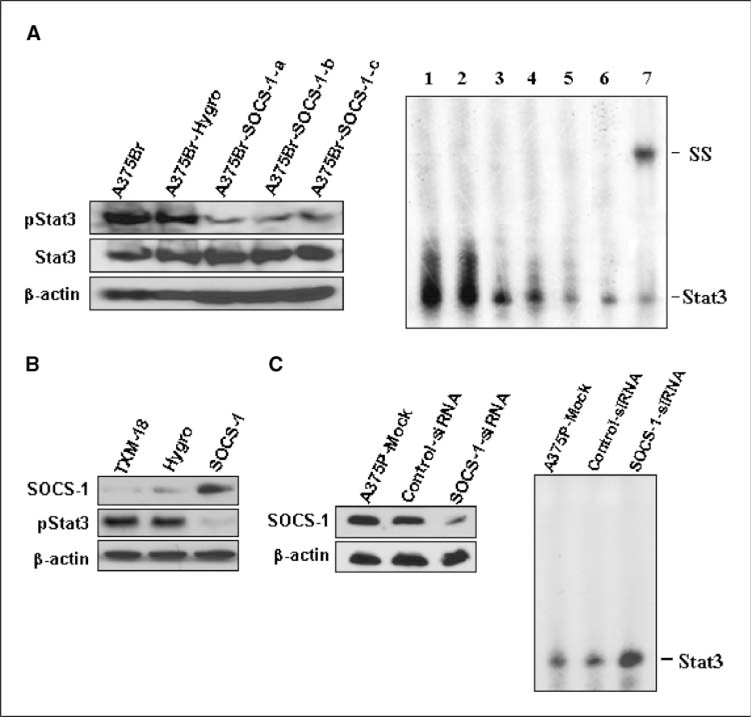
Figure 3.
Altering SOCS-1 expression affects Stat3 activation. A, left, Stat3 and tyrosine-phosphorylated Stat3 levels in A375Br, A375Br-hygro, and A375Br-SOCS-1 cells were determined by Western blotting. Right, restoring SOCS-1 inhibits the DNA-binding activity of Stat3 in A375Br cells, as determined by EMSA. Nuclear protein extracts (10 μg/sample) of A375Br (lane 1), A375Br-hygro (lane 2), or A375Br-SOCS-1-a, A375Br-SOCS-1-b, and A375Br-SOCS-1-c (lanes 3–5) cells were subjected to EMSA with 32P-labeled oligonucleotide probes containing a consensus-binding motif for Stat3. In some reactions with A375Br protein, unlabeled Stat3 consensus probe (lane 6) or anti-Stat3 antibody (lane 7) was added. The positions of the Stat3 and supershifted (SS) complexes were marked. B, restoring SOCS-1 expression inhibits Stat3 tryrosine phosphorylation in TXM-18 cells. TXM-18 cells were transfected with pcDNA-SOCS-1 or control plasmids twice in 48 h, and then cells were harvested for Western blot analysis of the expression of SOCS-1 and tyrosine-phosphorylated Stat3. C, left, depletion of SOCS-1 expression in zA375P cells by transfection with SOCS-1-siRNA. SOCS-1 expression in mock-transfected, control-siRNA–transfected, or SOCS-1-siRNA–transfected A375P cells was determined by Western blotting. Right, depletion of SOCS-1 increases Stat3 DNA-binding activity in A375P cells. Stat3 DNA-binding activity in mock-transfected, control-siRNA–transfected, or SOCS-1-siRNA–transfected A375P cells was determined by EMSA.