Figure 1.
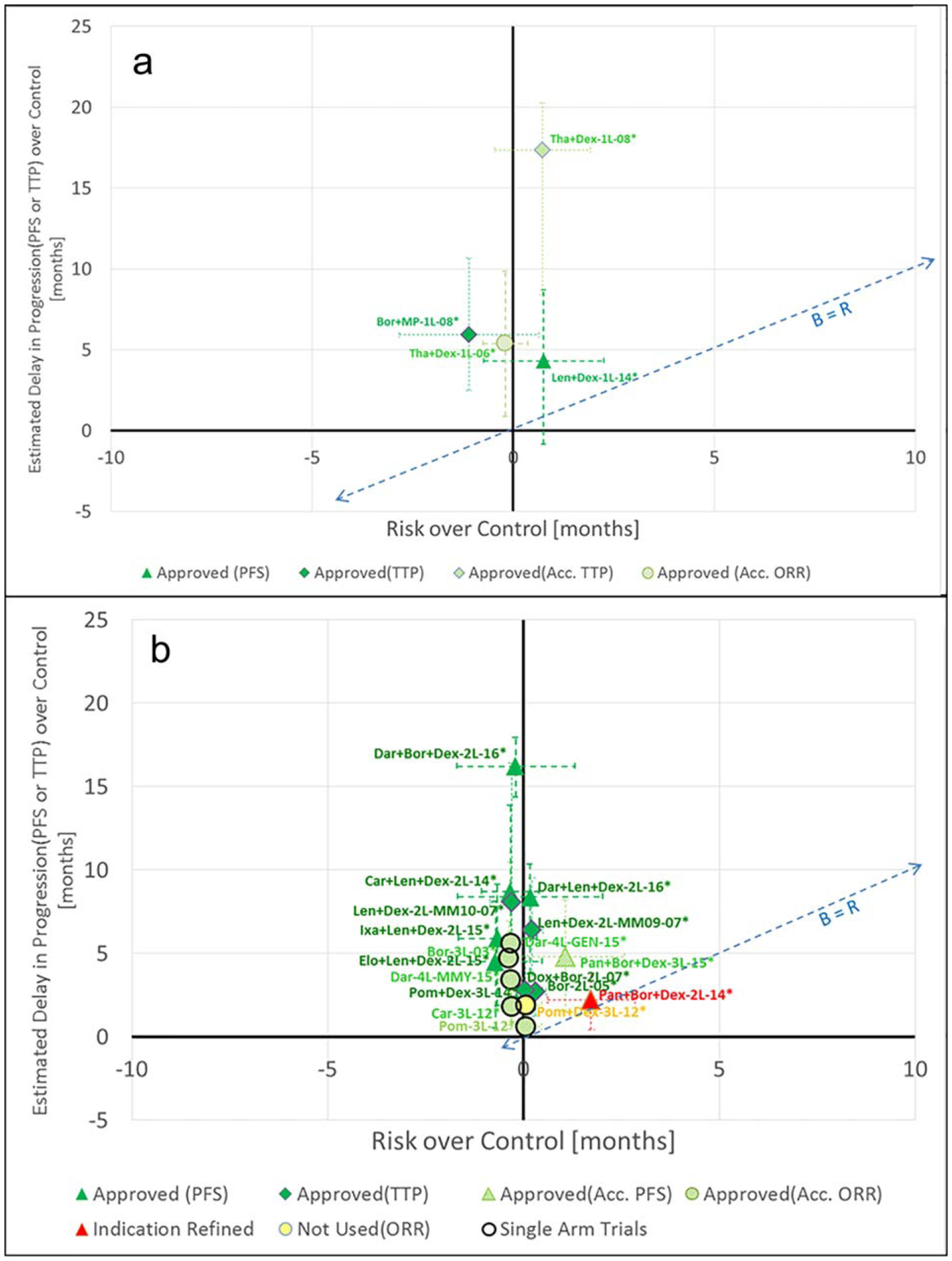
Analysis of benefits and risks (a). First-line (b). Nonfirst-line. The naming convention for the points in the chart above is as follows: NNN-LL QQQQ-YY; where: NNN represents the first three letters in the name of the drug (e.g., “Len” for lenalidomide; LL represents the line of treatment (e.g., 1L indicating lenalidomide used as the first-line of treatment); QQQQ (if needed) is a description of a relevant subpopulation and/or specific pivotal trial; YY represents the year associated with the study/decision pertaining to the data point. The PFS improvement shown for the point Pan+Bor+Dex-2L-14* in Figure 1b is based on an assessment by an Independent Review Committee (IRC).
