Figure 4.
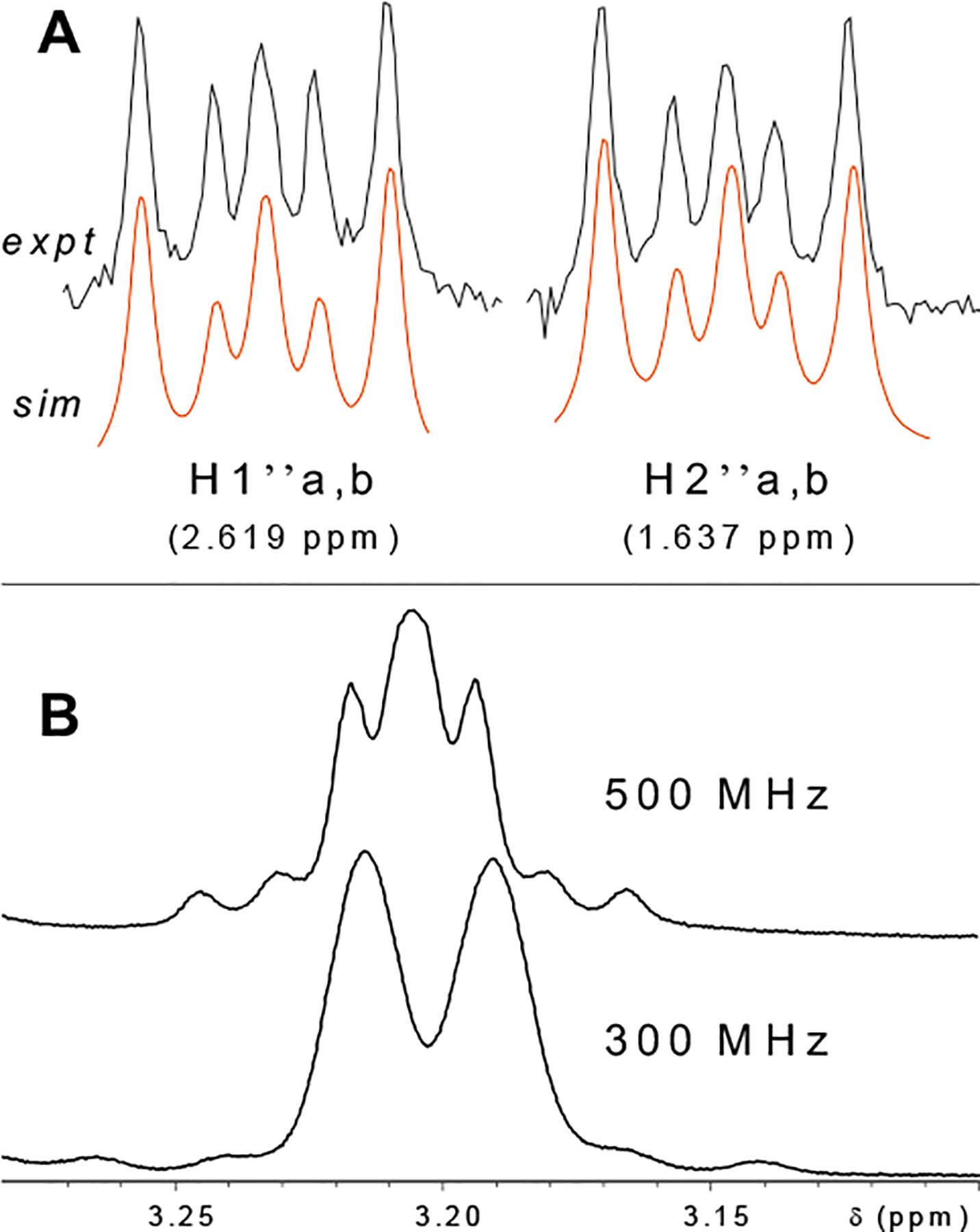
Unique signals diagnostic of partial structures of flavanoids from Humulus lupulus. Above (A) are signals for the AA′XX′ spin system comprised of the prenyl-methylene groups in xanthohumol H (5). The magnetic non-equivalence between the isochronic protons, indicating that this is not a freely-rotating system in MeOH solution at room temperature, is evidenced by the observation of geminal coupling. 2J = 16.00 Hz, 3Jgauche = 4.88 Hz, 3Jtrans=11.79 Hz. In B, the effect of spectrometer frequency on spin multiplicity of the H1″ prenyl-unit methylene signal is illustrated for the 8-prenylated flavanone, isoxanthohumol (17). At 300 MHz, Δv = 6 Hz for the two nuclei, whereas at 500 MHz Δv = 10 Hz. Since the geminal coupling constant of 13.8 Hz is independent of field strength, as spectrometer frequency decreases, the ratio J/Δv increases, resulting in a higher order spectrum. The same higher order effect was observed for the 5-O-demethyl derivative of 17, 8-prenylnaringenin (15). These data cannot be reproduced in tables without first conducting full spin system analyses.
