Abstract
There is no established postoperative adjuvant therapy for hepatocellular carcinoma (HCC), and improvement of patient prognosis has been limited. We conducted long‐term monitoring of patients within a phase II trial that targeted a cancer antigen, glypican‐3 (GPC3), specifically expressed in HCC. We sought to determine if the GPC3 peptide vaccine was an effective adjuvant therapy by monitoring disease‐free survival and overall survival. We also tracked GPC3 immunohistochemical (IHC) staining, CTL induction, and postoperative plasma GPC3 for a patient group that was administered the vaccine (n = 35) and an unvaccinated patient group that underwent surgery only (n = 33). The 1‐y recurrence rate after surgery was reduced by approximately 15%, and the 5‐y and 8‐y survival rates were improved by approximately 10% and 30%, respectively, in the vaccinated group compared with the unvaccinated group. Patients who were positive for GPC3 IHC staining were more likely to have induced CTLs, and 60% survived beyond 5 y. Vaccine efficacy had a positive relationship with plasma concentration of GPC3; high concentrations increased the 5‐y survival rate to 75%. We thus expect GPC3 vaccination in patients with HCC, who are positive for GPC3 IHC staining and/or plasma GPC3 to induce CTL and have significantly improved long‐term prognosis.
Keywords: cytotoxic T lymphocyte, glypican‐3, hepatocellular carcinoma, immunohistochemical staining, peptide vaccine
This phase II study demonstrated that postoperative adjuvant vaccination may reduce the 1‐y recurrence rate and prolong overall survival in patients who are positive for GPC3 IHC staining. The GPC3 peptide vaccine used induces specific CTLs and can be expected to improve patient prognosis.

Abbreviations
- DFS
disease‐free survival
- GPC3
glypican‐3
- HCC
hepatocellular carcinoma
- IHC staining
immunohistochemical staining
- OS
overall survival
1. INTRODUCTION
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections can induce a chronic inflammatory microenvironment in the liver and are considered major risk factors for hepatocellular carcinoma (HCC). 1 Drug development has made it possible to suppress the onset of viral chronic hepatitis and HCC. 2 However, with changes in lifestyle and associated increases in prevalence of non‐alcoholic fatty liver disease (NAFLD) and non‐alcoholic steatohepatitis (NASH), HCC still remains one of the leading causes of cancer‐related deaths globally. 3 , 4 Due to a high risk of metastasis and de novo development of tumors, the postoperative 5‐y recurrence rate of HCC exceeds 70%. 5 , 6 , 7 There are various options for HCC treatment other than surgery. These include radiofrequency ablation therapy (RFA), transarterial chemoembolization (TACE), and chemotherapies like sorafenib and lenvatinib. However, none of the above therapies has been effective at preventing relapse, and no effective postoperative adjuvant therapy has been established. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 Immunotherapy has the potential to be a treatment option for HCC. 23 , 24 Indeed, many decades of study have been invested in the development of immunotherapies against HCC, and many HCC immunotherapy clinical trials have been performed. 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 Several randomized controlled trials have suggested that the use of immunotherapy as an adjuvant therapy reduces the risk of cancer recurrence. 24 , 33 , 34 The carcinoembryonic antigen glypican‐3 (GPC3) is specifically overexpressed in 80% HCC and therefore is an ideal target for antigen‐specific immunotherapy. 35 , 36 , 37 , 38 While the functions of membrane‐anchored GPC3 remain unknown, it is likely to have a role in neoplastic transformation of HCC. 39 The expression of GPC3 in HCC has also been reported to promote tumor growth by activating Wnt pathway signaling and is associated with clinical diagnoses of intrahepatic metastasis and multicentric hepatocarcinogenesis. 40 , 41 , 42 , 43 We have identified peptides that bind to human leukocyte antigen (HLA)‐A24 or HLA‐A2 to induce GPC3 peptide‐specific CTLs, and have subsequently initiated clinical trials of vaccines based on these peptides. 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 We conducted a phase II study from 2009 to 2012 that used GPC3 peptide vaccine as an adjuvant therapy for HCC patients, and reported that the vaccine reduced the 1‐y recurrence rate in GPC3‐positive patients. 21 However, long‐term prognosis has not been studied in detail, and follow‐up beyond 5 y revealed that there were many long‐term survivors. Therefore, we sought to investigate the effect of postoperative adjuvant GPC3 peptide vaccine therapy on patient prognosis.
2. MATERIALS AND METHODS
2.1. Study design and participants
The phase II trial was carried out between September 2009 and August 2012. Patients with a diagnosis of HCC who had undergone surgery or RFA were enrolled. Inclusion and exclusion criteria for this study have been published previously. 21 The study was conducted with the formal approval of Ethics Committee of the National Cancer Center and conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The trial was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN‐CTR, UMIN000002614). Informed written consent was obtained from all study participants.
2.2. Sample collection and biomarker analysis
All liver specimens were prepared from surgically resected tumors. GPC3 IHC staining, CTL induction after vaccination, and plasma GPC3 were studied, as previously described. 47 , 52 , 53 Specimens were stained with hematoxylin and eosin or monoclonal antibodies (dilution 1:300) raised against GPC3 (clone 1G12; 1:200 dilution; BioMosaics) in accordance with the manufacturer’s instructions. An ex vivo interferon‐γ (IFN‐γ) ELISPOT assay was performed in duplicate and PBMCs (5 × 105/well) were treated with GPC3 peptide antigens to evaluate antigen‐specific CTL responses. The assay system for full‐length GPC3 was constructed using a sandwich immunoassay, in which a monoclonal mouse antibody against its N‐terminus was used to capture the protein and a monoclonal mouse antibody against the C‐terminus was used to detect the protein. The monoclonal antibody against the N‐terminus was labeled with biotin and the antibody against the C‐terminus with alkaline phosphatase. The immunoassay was performed by first reacting the plasma sample with the biotinylated antibody, then capturing the immunocomplex using streptavidin‐coated magnetic beads. After washing the beads, an alkaline phosphatase‐labeled antibody was added to form a sandwich immunocomplex. After a second wash, a luminescent substrate was added and the luminescence intensity was measured. All immunoassay steps were performed using an HISCLTM‐800 (Sysmex Co.), which is an automated immunoassay device. Recombinant GPC3 (R&D Systems Inc) was used as the assay standard. Standards at concentrations of 2, 15, 50, 150, 500, or 1500 pg/mL were measured and calibration curves were generated using the four‐parameter logistic regression method.
2.3. Statistical analysis
All statistical analyses were conducted using a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) known as EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan). A Kaplan‐Meier analysis was used to compare survival rates. A log‐rank test and Cox proportional hazard models were used to identify prognostic factors. Patient characteristics were compared using Pearson χ2 test, Student t test, and the Mann‐Whitney U test. The level of significance was set at P < .05.
3. RESULTS
3.1. Study population
This article is a follow‐up report; details on the main findings of the phase II study have been published previously. 21 The study included surgical and RFA cases, and only 35 patients who underwent surgery were evaluated in this report. As it was a single‐arm study conducted with 35 vaccinated surgical patients, they were compared with 33 control patients who underwent surgery without vaccination at our hospital during the same period. Of the vaccinated group, IHC staining could not be performed for 4 patients who underwent surgery at a different hospital. However, no significant differences were observed in patient background for both groups. In the previous report, the median observation period was 40.4 mo, but for the purpose of monitoring long‐term prognosis, the median observation period was extended to 72.8 mo for this study.
3.2. Effects of the GPC3 peptide vaccine on recurrence and survival
Comparing disease‐free survival (DFS) in vaccinated and unvaccinated patients, 1‐y postoperative recurrence rates were 25.7% and 42.4% respectively. This confirmed that the vaccine decreased recurrence. However, there was no difference (P = .83, log‐rank test) in long‐term recurrence rates beyond 5 y (Figure 1A). In contrast, long‐term overall survival (OS) was significantly higher in the vaccine group at 5 (70.6% vs 57.6%) and 8 y (67.1% vs 38.9%). Although the GPC3 peptide vaccine did not reduce long‐term recurrence rates, patients had a significant benefit (P = .038, log‐rank test) in terms of long‐term survival (Figure 1B). While it has been reported previously that there was no difference in OS, here, long‐term observation revealed the late effect that the peptide vaccine prolonged OS. 21
Figure 1.
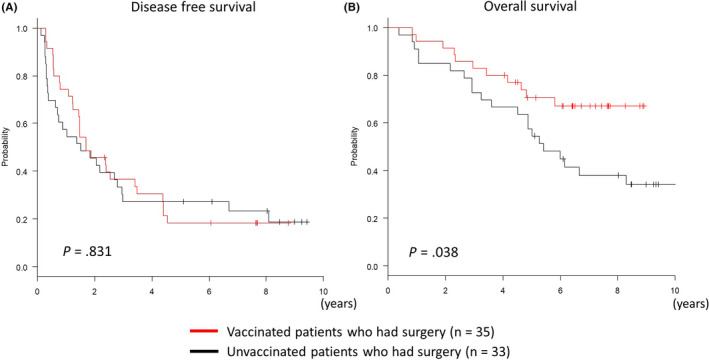
Kaplan‐Meier curves for disease‐free survival (DFS) and overall survival (OS). A, After surgery, DFS for patients who were vaccinated (n = 35) was not significantly different compared with that for unvaccinated patients (n = 33). B, The survival rate was significantly higher in the vaccinated group than in the unvaccinated group after 5 y (70.6% vs 57.6%) and 8 y (67.1% vs 38.9%)
3.3. Effect of GPC3 peptide vaccine in patients with recurrent HCC
In this study, 80% of the patients in this study relapsed regardless of administration of GPC3 peptide vaccine. However, long‐term survival was improved in the vaccinated group. We sought to determine if there were any differences in patients who had recurrence with the GPC3 peptide vaccine and those who did not. As described in Figure 1A, there was no significant difference in the time until the first recurrence. However, the time until the second recurrence from the first recurrence was significantly prolonged in the vaccinated group (Figure 2A; P = .011, log‐rank test), and the vaccinated group had better OS after the first recurrence than the unvaccinated group (Figure 2B; P = .012, log‐rank test). Therefore, even in cases with recurrence, the second recurrence was less likely to occur due to the GPC3 peptide vaccine. As a result, OS was considered to be prolonged in patients who were vaccinated. When patients who relapsed were categorized into 2 groups with different times to recurrence within 1 y or after 1 y, patients who had a recurrence within 1 y of surgery had significantly poorer prognosis (Figure 3A; P < .01, log‐rank test). Vaccination improved OS regardless of time to recurrence, and some patients survived long term beyond 5 y (Figure 3B). When multivariate analysis was performed using the Cox proportional hazards model, GPC3 peptide vaccination (hazard ratio (HR): 0.41, 95% confidence interval (CI): 0.19‐0.88, P = .023) and recurrence within 1 y (HR: 6.3, 95% CI: 2.8‐14.2, P < .01) were both significantly associated with OS. Therefore, we concluded that GPC3 peptide vaccination decreased 1‐y recurrence rates, and this could have contributed to improved patient prognosis.
Figure 2.
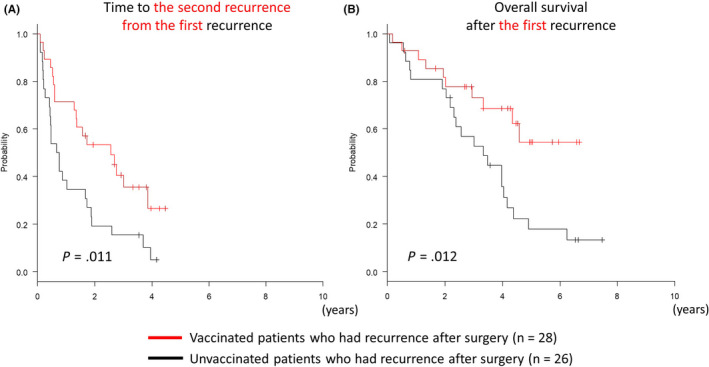
Kaplan‐Meier curves for the time until second recurrence from the first recurrence and OS after the first recurrence. A, Vaccinated patients (n = 28) showed a longer time to the second recurrence from the first recurrence after surgery compared with unvaccinated patients (n = 26). B, The vaccinated group had better prognosis 5 y after the first recurrence compared with the unvaccinated group
Figure 3.
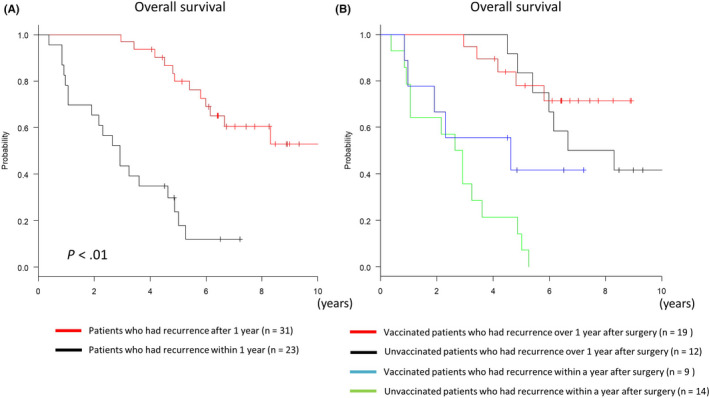
Kaplan‐Meier curves for overall survival (OS). A, Patients who had recurrence after 1 y (n = 31) had significantly better OS than patients who had a recurrence within 1 y (n = 23). B, OS improved in the vaccinated group regardless of whether recurrence took place within 1 y or later; some patients survived long term (beyond 5 y)
3.4. DFS in patients with GPC3‐positive or GPC3‐negative IHC staining
In the unvaccinated group, 1‐y recurrence rates of patients who had positive and negative GPC3 IHC staining were 52.4% and 25.0%, respectively (Figure 4A; P = .26, log‐rank test). Therefore, the GPC3‐positive group had a higher rate of relapse within 1 y compared with the negative group. In the GPC3‐positive group, vaccination reduced the 1‐y recurrence rate to the same level as found for the negative group; compared with that of the unvaccinated group, there was no statistical difference in recurrence rate (Figure 4A; P = .60, log‐rank test).
Figure 4.
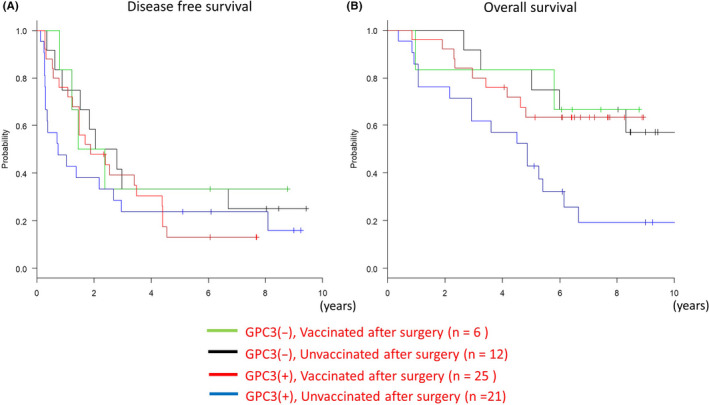
Kaplan‐Meier curves for disease‐free survival (DFS) and overall survival (OS). A, Glypican‐3 (GPC3)‐positive patients who were unvaccinated had a higher recurrence rate after 1 y compared with other groups. B, Prognosis for vaccinated patients in the GPC3‐positive group was as good as that for the GPC3‐negative group and better than that for the unvaccinated group
3.5. OS in patients with GPC3‐positive or GPC3‐negative IHC staining
For the unvaccinated group, 5‐y survival rates of patients whose samples stained positive or negative for GPC3 were 42.9% and 75.0%, respectively. The GPC3‐positive group had a significantly poorer prognosis (P = .021, log‐rank test) compared with the negative group (Figure 4B). The GPC3‐negative group had a good prognosis regardless of vaccine administration. In the GPC3‐positive group, the prognosis of patients who had been vaccinated was improved and was equivalent to that of unvaccinated patients in the GPC3‐negative group (Figure 4B; P = .014, log‐rank test).
3.6. GPC3 IHC staining and CTL induction
CTL induction was examined to study the effect of vaccination. To determine if the GPC3 peptide vaccine could induce a specific immune response, an ex vivo IFN‐γ ELISPOT assay was performed using PBMCs obtained from all patients before and after vaccination. In both the GPC3‐negative and GPC3‐positive groups, peptide‐specific CTL were difficult to detect in the blood before vaccination because the levels were below the detection limit. After vaccination, CTL number ranged from 1 to 648 with a median of 27. When the number of CTL induced was examined separately by GPC3 IHC staining, the maximum number induced was higher in the GPC3‐positive group (648) compared with the GPC3‐negative group (79). Average induction number was also higher in the GPC3‐positive group (106) compared with that in the GPC3‐negative group (25). Positive induction was defined as the median of the top 3 mean values of CTL measured after vaccination, or ≥27 CTL. Using these criteria, positive induction of CTL occurred in 16.7% of patients in the GPC3‐negative group and 60.0% in the GPC3‐positive group (Table 1; P = .083, Fisher exact test). In the GPC3‐positive group, an immune response to the GPC3 peptide antigen had already occurred in the past, and precursor cells of CTL were present in the patient's body. Therefore, it was considered that vaccination facilitated induction of CTL. The relationship between the number of CTL induced and OS for groups with different GPC3 IHC staining is shown in Figure 5A. Induction of CTL alone did not lead to good OS, but the results of GPC3 IHC staining were also relevant. In the GPC3‐positive group, 15/25 patients (60%) had OS that exceeded 5 y (long term) and 11 patients of 15 long‐term survivors (73%) were positive for CTL induction. Conversely, in the GPC3‐positive group, 6/25 cases (24%) were negative and had no improvement in OS. In the GPC3‐negative group, 5/6 cases (83.3%) survived beyond 5 y, and survival was high regardless of CTL induction. Plasma GPC3 concentration was measured 1‐mo post‐operation, and a concentration of ≥6.8 pg/mL was defined as positive for plasma GPC3. 52 When postoperative plasma levels of GPC3 were included in the analysis, 12 patients were found to be positive for GPC3 in plasma and IHC staining and, in this group, OS beyond 5 y was shown in 9/12 (75%) patients (Figure 5B). Here, 7 of 9 (77.8%) patients who survived beyond 5 y were CTL positive, and 1 patient remained alive within 5 y. There were no drop‐outs due to death in the 8 CTL‐positive patients (Figure 6A). There were 13 patients who were positive for GPC3 IHC staining and negative for postoperative plasma GPC3. In this group, the 5‐y survival rate for 7 CTL‐positive patients was 57.1%, but prognosis was poor for the remaining 6 CTL‐negative patients with a lower (33.3%) 5‐y survival rate (Figure 6B). In the GPC3‐negative group, 5/6 cases (83.3%) survived beyond 5 y, regardless of postoperative plasma GPC3 levels (Figure 5B).
Table 1.
Relationship between glypican‐3 (GPC3) immunohistochemical (IHC) staining status and CTL induction
| GPC3 IHC staining (+/−) | CTL maximum numbers | CTL average numbers | CTL median (range) | CTL positive (CTL ≥ 27) | CTL negative (CTL < 27) |
|---|---|---|---|---|---|
| + | 648 | 106 | 27 (1‐648) | 15/25 (60.0%) | 10/25 (40.0%) |
| − | 79 | 25 | 1/6 (16.7%) | 5/6 (83.3%) |
Figure 5.
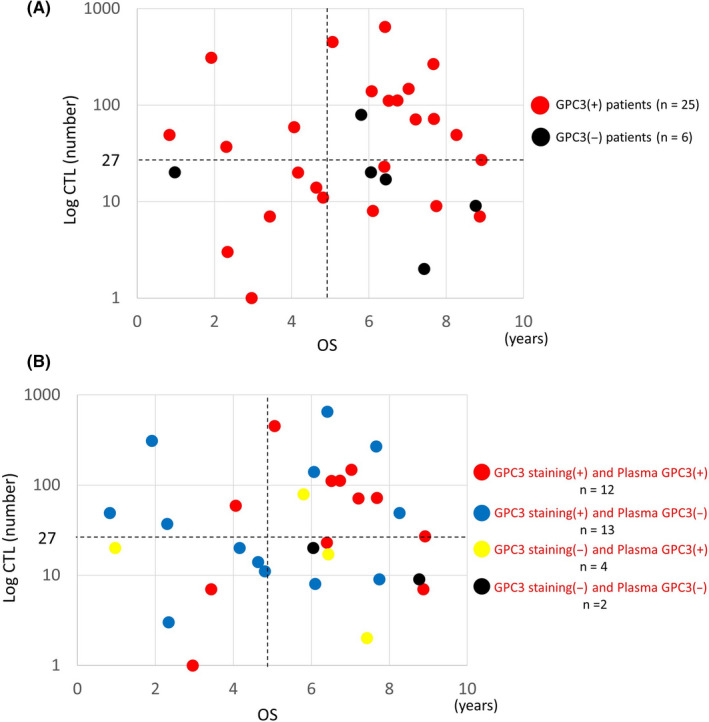
Relationship between CTL number, overall survival (OS), glypican‐3 (GPC3) immunohistochemical (IHC) staining and plasma GPC3. A, In the GPC3‐positive group, 15/25 cases (60%) were long‐term (beyond 5 y) survivors. Of the long‐term survivors, 11/15 (73%) had CTL induction. B, Of the patients who were positive for both GPC3 IHC staining and plasma GPC3 (n = 12), 9/12 (75%) were long‐term (beyond 5 y) survivors. Of the long‐term survivors, 7/9 (77.8%) had CTL induction
Figure 6.
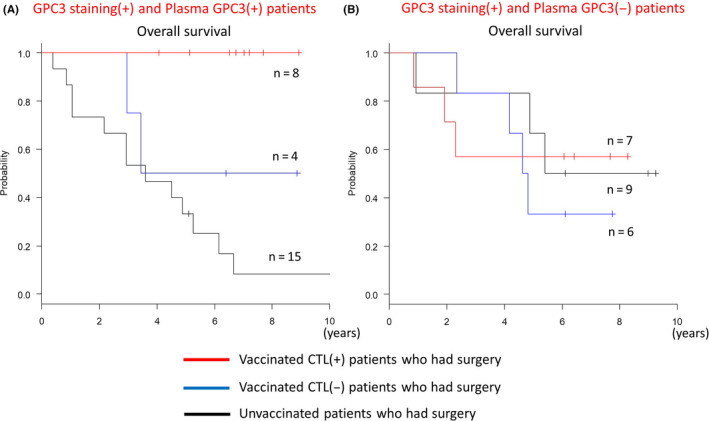
Kaplan‐Meier curves for overall survival (OS). A, There were no drop‐outs due to death in patients who were positive for glypican‐3 (GPC3) immunohistochemical (IHC) staining, plasma GPC3, and CTL induction (n = 8). B, There were patients who were positive for GPC3 IHC staining and negative for postoperative plasma GPC3 (n = 13). Prognosis for patients with CTL induction (n = 7) was good, with a 5‐y survival rate of 57.1%
Plasma GPC3 levels were measured for both vaccinated and unvaccinated groups (Figure 7). Among patients who were GPC3 positive for IHC staining, vaccinated patients who maintained high levels of plasma GPC3 tended to have good long‐term survival (Figure 7A), but in the unvaccinated group, patients with a high level of plasma GPC3 had poorer survival (Figure 7B). Regardless of vaccination status, long‐term survivors who were GPC3 negative by IHC staining often had only a slight increase in plasma GPC3 levels (Figure 7C,D). Postoperative persistent plasma GPC3 suggested that there were residual cancer cells. Therefore, it was surprising that vaccinated patients who were positive for GPC3 by IHC staining had increased long‐term survival despite increased plasma GPC3 levels. For patients who were GPC3 positive in IHC staining, OS was poor when plasma GPC3 was high in the unvaccinated group (Figure 8A). However, in the vaccinated group, an opposite association was observed, and OS was improved when plasma GPC3 was high (Figure 8B). In patients who had GPC3‐positive IHC staining, an increase in plasma GPC3 concentration carried completely different meanings for the vaccinated and unvaccinated groups. As the vaccine suppresses recurrence and improves OS, we believe that this is strong evidence that higher plasma GPC3 levels increased patient responses to the GPC3 antigen in the vaccine. For individual patients, most of the 16 patients who were long‐term survivors and had GPC3‐positive IHC staining also had higher levels of plasma GPC3 and were more likely to have CTL induced (Figure 9A). Of the 9 dead patients, a majority had an early relapse with low levels of plasma GPC3 and low CTL induction (Figure 9B). Characteristics of the patients are represented in Table 2. As previously reported, GPC3‐positive IHC staining indicated poor prognosis in patients, but vaccinated patients had improved OS because GPC3 peptide vaccines were more likely to increase plasma GPC3 levels and promote CTL induction.
Figure 7.
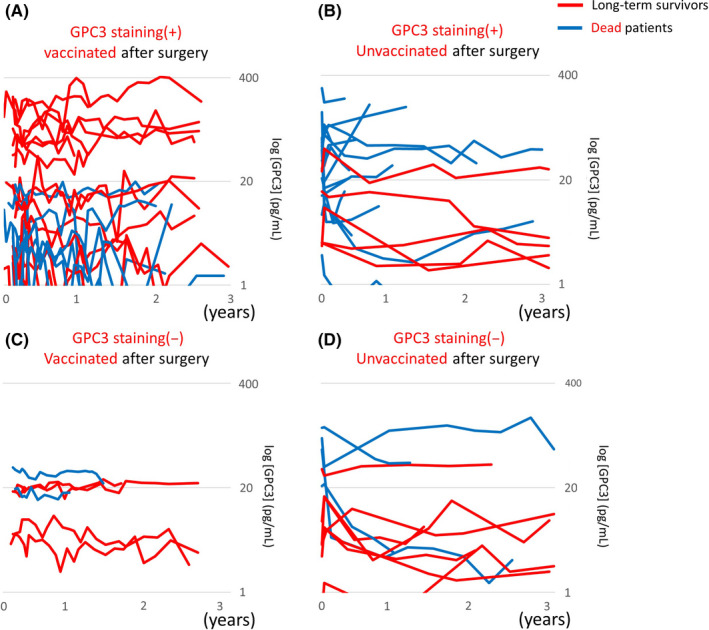
Changes in plasma glypican‐3 (GPC3) according to GPC3 IHC staining and the vaccination. Long‐term survivors are indicated by red lines, and dead patients are indicated by blue lines. A, Vaccinated patients who were GPC3 immunohistochemical (IHC) staining positive and maintained high plasma GPC3 levels tended to have increased long‐term survival. B, High plasma GPC3 levels were associated with death in the GPC3 IHC staining positive and unvaccinated group. C, Long‐term survivors who were GPC3 IHC staining negative often only had a slight increase in plasma GPC3 levels. D, High plasma GPC3 levels were associated with death in the GPC3 IHC staining negative and unvaccinated group
Figure 8.
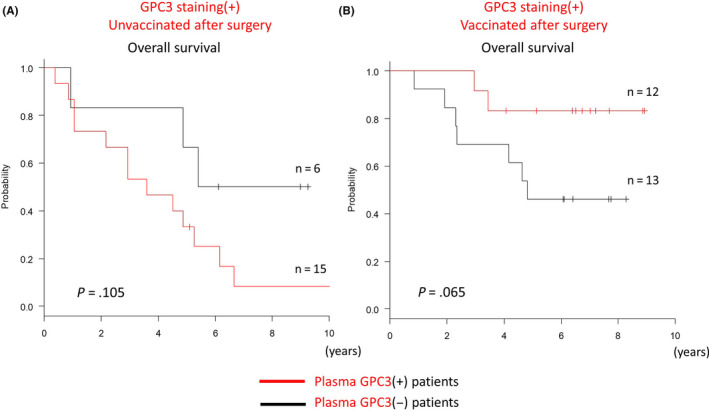
Kaplan‐Meier curves for overall survival (OS) in patients who were positive for GPC3 immunohistochemical (IHC) staining. A, In the unvaccinated group, OS was poor when plasma GPC3 was high. B, In the vaccinated group, OS was increased in patients with high levels of plasma GPC3
Figure 9.
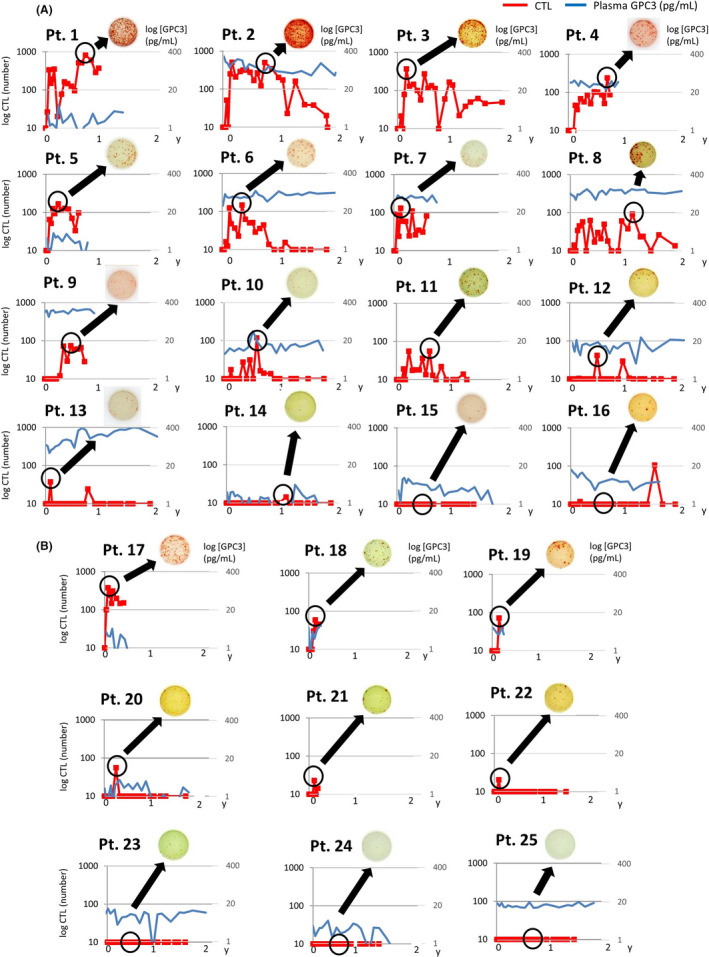
CTL induction and plasma glypican‐3 (GPC3) levels in individual patients. A representative CTL induction image obtained by ELISpot assay is shown. A, Long‐term survivors who were positive for GPC3 IHC staining (n = 16) had higher levels of plasma GPC3 and were more likely to have CTL induction. B, Of the dead patients (n = 9), a majority had an early relapse with low levels of plasma GPC3 and low CTL induction
Table 2.
A, Characteristics of glypican‐3 (GPC3) IHC staining (+) long‐term survivors. B, Characteristics of GPC3 staining (+) patients who are dead
| No. | Age/sex | GPC3 IHC staining | Plasma GPC3 (pg/mL) | CTL (number) | Recurrence | DFS (y) | OS (y) | |
|---|---|---|---|---|---|---|---|---|
| A, | ||||||||
| 1 | 62 | M | + | 3.1 | 648 | + | 1.5 | 6.4 |
| 2 | 70 | M | + | 267.3 | 452 | + | 4.6 | 5.1 |
| 3 | 51 | M | + | 0.5 | 267 | − | – | 7.7 |
| 4 | 60 | M | + | 42.8 | 148 | + | 1.1 | 7.0 |
| 5 | 47 | F | + | 2.5 | 139 | − | – | 6.1 |
| 6 | 60 | M | + | 30.6 | 112 | + | 2.6 | 6.7 |
| 7 | 48 | M | + | 57.1 | 111 | + | 0.8 | 6.5 |
| 8 | 65 | F | + | 91.4 | 72 | − | – | 7.7 |
| 9 | 78 | F | + | 187.3 | 71 | + | 0.5 | 7.2 |
| 10 | 64 | M | + | 7.1 | 59 | + | 2.5 | 4.1 |
| 11 | 71 | M | + | 1.7 | 49 | + | 3.4 | 8.3 |
| 12 | 75 | F | + | 17.9 | 27 | + | 4.4 | 8.9 |
| 13 | 56 | M | + | 229.4 | 23 | + | 3.5 | 6.4 |
| 14 | 61 | M | + | 2.3 | 9 | + | 4.4 | 7.7 |
| 15 | 60 | M | + | 2.9 | 8 | + | 3.4 | 6.1 |
| 16 | 63 | M | + | 14.8 | 7 | + | 4.4 | 8.9 |
| B, | ||||||||
| 17 | 49 | F | + | 3.7 | 309 | + | 0.6 | 1.9 |
| 18 | 61 | M | + | 5.4 | 49 | + | 0.3 | 0.8 |
| 19 | 68 | F | + | 6.1 | 37 | + | 0.3 | 2.3 |
| 20 | 75 | M | + | 1.9 | 20 | + | 1.2 | 4.2 |
| 21 | 55 | M | + | 0.5 | 14 | + | 0.3 | 4.6 |
| 22 | 69 | M | + | 0.5 | 11 | + | 1.5 | 4.8 |
| 23 | 78 | M | + | 10.7 | 7 | + | 1.5 | 3.4 |
| 24 | 77 | M | + | 4.0 | 3 | − | – | 2.3 |
| 25 | 75 | F | + | 18.2 | 1 | + | 1.9 | 3.0 |
4. DISCUSSION
Hepatocellular carcinoma is known to have a high recurrence rate of approximately 70%, which makes it difficult to treat and consequently is associated with poor prognosis. 5 , 6 , 7 In our previously reported phase II study, primary endpoints were 1‐y and 2‐y recurrence rates and expected recurrence rates were 20% and 45%, respectively. 21 Overall, as we observed recurrence rates of 25.7% and 54.3%, we concluded that the vaccine, as with many other adjuvant treatments including sorafenib, failed to prevent recurrence. 34 However, in patients who were positive for IHC staining, the 1‐y recurrence rates of the vaccinated and unvaccinated groups were 24.0% and 52.4% respectively. Therefore, we concluded that vaccination in the group that was positive for IHC staining reduced the 1‐y recurrence rate, and could prevent early recurrence after surgery. We hypothesized that a decrease in 1‐y recurrence rate (Figure 3A,B) might also lead to improvement in long‐term OS and, indeed, there were long‐term survivors in this study.
Several clinical trials have previously reported that immunotherapy improves prognosis of postoperative HCC patients, however our previous report did not include a long‐term follow‐up. 23 , 24 , 54 As DFS often does not correlate with OS and cannot be a surrogate readout for OS, long‐term follow‐up is particularly important for immunotherapy. 55 While vaccination reduced the 1‐y recurrence rate for GPC3‐positive patients, another major benefit for the vaccinated group was that recurrence occurred later compared with that in the unvaccinated group (Figure 2A). Thus, even for patients who had relapsed, the vaccinated group had a better prognosis after recurrence compared with the unvaccinated group (Figure 2B). Numerous reports have suggested that administration of a GPC3 peptide vaccine induces GPC3‐specific CTL, and that infiltration of CTL into the tumors produces an anti‐tumor effect. 21 , 45 , 47 , 48 , 49 , 56 , 57 We examined the relationship between clinical efficacy of the postoperative adjuvant vaccine with induction of CTL and GPC3 IHC staining (Table 1). As GPC3 IHC staining of tissue obtained from resected tumor specimen reflects the characteristics of the tumor itself, this test is predictive of CTL induction and vaccine efficacy. Various types of GPC3 IHC staining in cells have been described. This includes GPC3 staining in the cytoplasm and cell membrane (diffuse type), uniform staining of the cell membrane (membrane type), and granular staining of the cell membrane (granule type). As there are various conflicting opinions regarding staining classifications, for this study, staining types or localization was not examined, and GPC3 IHC staining was simply classified as positive or negative. 33 , 43 , 50 , 58 , 59 , 60 , 61 , 62 It has previously been reported that following initial hepatectomy, prognosis for GPC3‐negative patients is good and that for GPC3‐positive patients is poor. 43 , 63 , 64 , 65 Consistent with these reports, we also observed that the GPC3‐negative group had good OS regardless of vaccine administration (Figure 2B). While poor prognosis was expected for GPC3‐positive patients, surprisingly, prognosis for vaccinated patients who were GPC3 positive improved and was comparable with that of the GPC3‐negative group. It is likely that the mechanism for this involves CTL induced by the GPC3 peptide vaccine administration (Figure 3A).
Plasma GPC3 was considered to be produced by viable cancer cells. Accordingly, the unvaccinated patient group with high plasma GPC3 had a poor prognosis because of the presence of viable cancer cells in the body. Vaccinated and unvaccinated plasma GPC3‐negative patients, who were not considered to have viable cancer cells remaining, showed a similar survival (Figure 8A,B). Conversely, vaccinated patients with high plasma GPC3 had a better prognosis, which indicated the presence of viable cancer cells in the body. Because plasma GPC3 levels near the cut‐off value were difficult to interpret, the group with high plasma GPC3 was further analyzed. The criterion for high plasma GPC3 was ≥15.8, which was analyzed using the Youden index for DFS (AUC: 0.853, 95% CI: 0.68‐1) and OS (AUC: 0.762, 95% CI: 0.529‐0.996) in the surgery alone group, was not affected by the vaccine. When DFS and OS were analyzed based on this criterion, both were increased (P = .051 and P < .01). Vaccination could delay the time to recurrence, which was thought to lead to good OS (Figure S1). Observation of changes in plasma GPC3 on a case‐by‐case basis showed that, in the surgery alone group, plasma GPC3 increased to a high level, and many cases experienced recurrence. In the vaccine group, plasma GPC3 was high in some patients, but it decreased or remained flat with recurrence in only some patients (Figure S2A,B). It was possible that vaccination induced CTL, which were subsequently in continuous equilibrium with viable cancer cells, thus suppressing tumor growth.
Plasma GPC3 levels have also been linked to viral hepatitis such as hepatitis C, and they may potentially be used in the future for HCC surveillance management. 52 When both GPC3 IHC staining and postoperative plasma GPC3 were positive, CTL induction by the vaccine increased from 53% to 70% (Figure 3B), with an associated enhancement in response to the GPC3 antigen. To our knowledge, there are no other postoperative adjuvant treatments for HCC that have comparable efficacy. To increase efficacy of the GPC3 peptide vaccine, it will likely be used in combination with immune checkpoint inhibitors such as anti‐PD‐1 and anti‐PD‐L1 antibodies. 66 , 67 , 68 , 69 The GPC3 peptide vaccine has the ability to induce peptide‐specific CTL and change “cold tumors” to “hot tumors.” A combination of the peptide vaccine and an immune checkpoint inhibitor would also be expected to prevent recurrence and act on recurrent tumors, thereby improving prognosis. 70
Our ability to evaluate the efficacy of the vaccine was limited, as this was not a randomized controlled study, but rather a case‐control study. However, long‐term observations showed that OS can be prolonged by vaccine administration. Combination with conventional chemotherapy or other immunotherapies such as immune checkpoint inhibitors may give rise to new treatment options.
In conclusion, this case‐control study demonstrated that postoperative adjuvant vaccination may reduce the 1‐y recurrence rate and prolong OS in patients who are positive for GPC3 IHC staining. The GPC3 peptide vaccine used induces specific CTLs and can be expected to improve patient prognosis.
DISCLOSURE
Tetsuya Nakatsura is a founder of and current shareholder at Killer T Save You Co., Ltd. TN is currently receiving royalties from Onco Therapy Science, Inc and fundamental research funding support from Thyas Co., Ltd. and Sysmex Co., Ltd. The remaining authors declare that they have no commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
Figure S1
Figure S2
ACKNOWLEDGMENTS
The authors would like to thank Kayoko Shoda and Yukiko Kozaki for their technical assistance. We would like to thank Editage (www.editage.jp) for English language editing. This work was supported by the National Cancer Center Research and Development Fund under Grant [25‐A‐7 and 28‐A‐8]; and by joint research funding from Thyas Co., Ltd. and Sysmex Co., Ltd.
Taniguchi M, Mizuno S, Yoshikawa T, et al. Peptide vaccine as an adjuvant therapy for glypican‐3‐positive hepatocellular carcinoma induces peptide‐specific CTLs and improves long prognosis. Cancer Sci. 2020;111:2747–2759. 10.1111/cas.14497
Trial registration: UMIN‐CTR, UMIN000002614
REFERENCES
- 1. Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol. 2014;28:753‐770. [DOI] [PubMed] [Google Scholar]
- 2. Carrat F, Fontaine H, Dorival C, et al. Clinical outcomes in patients with chronic hepatitis C after direct‐acting antiviral treatment: a prospective cohort study. Lancet. 2019;393:1453‐1464. [DOI] [PubMed] [Google Scholar]
- 3. Tokushige K, Hyogo H, Nakajima T, et al. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease and alcoholic liver disease: multicenter survey. J Gastroenterol. 2016;51:586‐596. [DOI] [PubMed] [Google Scholar]
- 4. Schutte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma‐epidemiological trends and risk factors. Dig Dis. 2009;27:80‐92. [DOI] [PubMed] [Google Scholar]
- 5. Hanazaki K, Kajikawa S, Shimozawa N. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg. 2000;191:381‐388. [DOI] [PubMed] [Google Scholar]
- 6. Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10‐year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumada T, Nakano S, Takeda I, et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology. 1997;25:87‐92. [DOI] [PubMed] [Google Scholar]
- 8. Osaki Y, Nishikawa H. Treatment for hepatocellular carcinoma in Japan over the last three decades: our experience and published work review. Hepatol Res. 2015;45:59‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Y, Deng T, Zeng LI, et al. Efficacy and safety of radiofrequency ablation and transcatheter arterial chemoembolization for treatment of hepatocellular carcinoma: a meta‐analysis. Hepatol Res. 2016;46:58‐71. [DOI] [PubMed] [Google Scholar]
- 10. Ulahannan SV, Duffy AG, McNeel TS, et al. Earlier presentation and application of curative treatments in hepatocellular carcinoma. Hepatology. 2014;60:1637‐1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng A‐L, Kang Y‐K, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia‐Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double‐blind, placebo‐controlled trial. Lancet Oncol. 2009;10:25‐34. [DOI] [PubMed] [Google Scholar]
- 12. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378‐390. [DOI] [PubMed] [Google Scholar]
- 13. Morimoto M, Numata K, Kondo M, et al. Higher discontinuation and lower survival rates are likely in elderly Japanese patients with advanced hepatocellular carcinoma receiving sorafenib. Hepatol Res. 2011;41:296‐302. [DOI] [PubMed] [Google Scholar]
- 14. Llovet JM, Villanueva A, Lachenmayer A, et al. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12:436. [DOI] [PubMed] [Google Scholar]
- 15. Chuma M, Terashita K, Sakamoto N. New molecularly targeted therapies against advanced hepatocellular carcinoma: from molecular pathogenesis to clinical trials and future directions. Hepatol Res. 2015;45:E1‐E11. [DOI] [PubMed] [Google Scholar]
- 16. Ikemoto T, Shimada M, Yamada S. Pathophysiology of recurrent hepatocellular carcinoma after radiofrequency ablation. Hepatol Res. 2017;47:23‐30. [DOI] [PubMed] [Google Scholar]
- 17. Cheng A‐L, Kang Y‐K, Lin D‐Y, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067‐4075. [DOI] [PubMed] [Google Scholar]
- 18. Ikeda K, Kudo M, Kawazoe S, et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52:512‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first‐line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non‐inferiority trial. Lancet. 2018;391:1163‐1173. [DOI] [PubMed] [Google Scholar]
- 20. Llovet JM, Decaens T, Raoul J‐L, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK‐PS study. J Clin Oncol. 2013;31:3509‐3516. [DOI] [PubMed] [Google Scholar]
- 21. Sawada YU, Yoshikawa T, Ofuji K, et al. Phase II study of the GPC3‐derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016;5:e1129483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hui D, Qiang LI, Jian W, et al. A randomized, controlled trial of postoperative adjuvant cytokine‐induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis. 2009;41:36‐41. [DOI] [PubMed] [Google Scholar]
- 23. Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802‐807. [DOI] [PubMed] [Google Scholar]
- 24. Kuang M, Peng BG, Lu MD, et al. Phase II randomized trial of autologous formalin‐fixed tumor vaccine for postsurgical recurrence of hepatocellular carcinoma. Clin Cancer Res. 2004;10:1574‐1579. [DOI] [PubMed] [Google Scholar]
- 25. Li S, Yang F, Ren X. Immunotherapy for hepatocellular carcinoma. Drug Discov Ther. 2015;9:363‐371. [DOI] [PubMed] [Google Scholar]
- 26. Yutani S, Shirahama T, Muroya D, et al. Feasibility study of personalized peptide vaccination for hepatocellular carcinoma patients refractory to locoregional therapies. Cancer Sci. 2017;108:1732‐1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun Z, Zhu Y, Xia J, et al. Status of and prospects for cancer vaccines against hepatocellular carcinoma in clinical trials. Biosci Trends. 2016;10:85‐91. [DOI] [PubMed] [Google Scholar]
- 28. Greten TF, Manns MP, Korangy F. Immunotherapy of HCC. Rev Recent Clin Trials. 2008;3:31‐39. [DOI] [PubMed] [Google Scholar]
- 29. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681‐700. [DOI] [PubMed] [Google Scholar]
- 30. Shi F, Shi M, Zeng Z, et al. PD‐1 and PD‐L1 upregulation promotes CD8(+) T‐cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;4:887‐896. [DOI] [PubMed] [Google Scholar]
- 31. Flecken T, Schmidt N, Hild S, et al. Immunodominance and functional alterations of tumor‐associated antigen‐specific CD8+ T‐cell responses in hepatocellular carcinoma. Hepatology. 2014;4:1415‐1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Breous E, Thimme R. Potential of immunotherapy for hepatocellular carcinoma. J Hepatol. 2011;4:830‐834. [DOI] [PubMed] [Google Scholar]
- 33. Peng BG, Liang LJ, He Q, et al. Tumor vaccine against recurrence of hepatocellular carcinoma. World J Gastroenterol. 2005;11:700‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double‐blind, placebo‐controlled trial. Lancet Oncol. 2015;16:1344‐1354. [DOI] [PubMed] [Google Scholar]
- 35. Nakatsura T, Yoshitake Y, Senju S, et al. Glypican‐3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun. 2003;306:16‐25. [DOI] [PubMed] [Google Scholar]
- 36. Shimizu Y, Suzuki T, Yoshikawa T, et al. Next‐generation cancer immunotherapy targeting glypican‐3. Front Oncol. 2019;9:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsuchiya N, Hosono A, Yoshikawa T, et al. Phase I study of glypican‐3‐derived peptide vaccine therapy for patients with refractory pediatric solid tumors. Oncoimmunology. 2017;7:e1377872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shimizu Y, Suzuki T, Yoshikawa T, et al. Cancer immunotherapy‐targeted glypican‐3 or neoantigens. Cancer Sci. 2018;109:531‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Capurro M, Wanless IR, Sherman M, et al. Glypican‐3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89‐97. [DOI] [PubMed] [Google Scholar]
- 40. Gattinoni L, Powell DJ, Rosenberg SA, et al. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Capurro MI, Xiang Y‐Y, Lobe C, et al. Glypican‐3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65:6245‐6254. [DOI] [PubMed] [Google Scholar]
- 42. Kikuchi A, Yamamoto H. Tumor formation due to abnormalities in the β‐catenin‐independent pathway of Wnt signaling. Cancer Sci. 2008;99:202‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shirakawa H, Suzuki H, Shimomura M, et al. Glypican‐3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci. 2009;100:1403‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nakatsura T, Komori H, Kubo T, et al. Mouse homologue of a novel human oncofetal antigen, glypican‐3, evokes T‐cell‐mediated tumor rejection without autoimmune reactions in mice. Clin Cancer Res. 2004;10:8630‐8640. [DOI] [PubMed] [Google Scholar]
- 45. Tsuchiya N, Yoshikawa T, Fujinami N, et al. Immunological efficacy of glypican‐3 peptide vaccine in patients with advanced hepatocellular carcinoma. Oncoimmunology. 2017;6:e1346764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Komori H, Nakatsura T, Senju S, et al. Identification of HLA‐A2‐ or HLA‐A24‐restricted CTL epitopes possibly useful for glypican‐3‐specific immunotherapy of hepatocellular carcinoma. Clin Cancer Res. 2006;12:2689‐2697. [DOI] [PubMed] [Google Scholar]
- 47. Yoshikawa T, Nakatsugawa M, Suzuki S, et al. HLA‐A2‐restricted glypican‐3 peptide‐specific CTL clones induced by peptide vaccine show high avidity and antigen‐specific killing activity against tumor cells. Cancer Sci. 2011;102:918‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sawada Y, Yoshikawa T, Nobuoka D, et al. Phase I trial of a glypican‐3‐derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res. 2012;18:3686‐3696. [DOI] [PubMed] [Google Scholar]
- 49. Sawada YU, Yoshikawa T, Fujii S, et al. Remarkable tumor lysis in a hepatocellular carcinoma patient immediately following glypican‐3‐derived peptide vaccination: an autopsy case. Hum Vaccin Immunother. 2013;9:1228‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Motomura Y, Ikuta Y, Kuronuma T, et al. HLA‐A2 and ‐A24‐restricted glypican‐3‐derived peptide vaccine induces specific CTLs: preclinical study using mice. Int J Oncol. 2008;32:985‐990. [PubMed] [Google Scholar]
- 51. Shafizadeh N, Ferrell LD, Kakar S. Utility and limitations of glypican‐3 expression for the diagnosis of hepatocellular carcinoma at both ends of the differentiation spectrum. Mod Pathol. 2008;21:1011‐1018. [DOI] [PubMed] [Google Scholar]
- 52. Shimizu Y, Mizuno S, Fujinami N, et al. Plasma and tumoral glypican‐3 levels are correlated in patients with hepatitis C virus‐related hepatocellular carcinoma. Cancer Sci. 2020;111:334‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Miura M, Fujinami N, Shimizu Y, et al. Usefulness of plasma full‐length glypican‐3 as a predictive marker of hepatocellular carcinoma recurrence after radial surgery. Oncol Lett. 2020;19:2657‐2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pan KE, Li Y‐Q, Wang W, et al. The efficacy of cytokine‐induced killer cell infusion as an adjuvant therapy for postoperative hepatocellular carcinoma patients. Ann Surg Oncol. 2013;20:4305‐4311. [DOI] [PubMed] [Google Scholar]
- 55. Tan A, Porcher R, Crequit P, et al. Differences in treatment effect size between overall survival and progression‐free survival in immunotherapy trials: a meta‐epidemiologic study of trials with results posted at clinicaltrials.gov. J Clin Oncol. 2017;35:1686‐1694. [DOI] [PubMed] [Google Scholar]
- 56. Suzuki S, Sakata J, Utsumi F, et al. Efficacy of glypican‐3‐derived peptide vaccine therapy on the survival of patients with refractory ovarian clear cell carcinoma. Oncoimmunology. 2016;5:e1238542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Suzuki S, Yoshikawa T, Hirosawa T, et al. Glypican‐3 could be an effective target for immunotherapy combined with chemotherapy against ovarian clear cell carcinoma. Cancer Sci. 2011;102:1622‐1629. [DOI] [PubMed] [Google Scholar]
- 58. Wang FH, Yip YC, Zhang M, et al. Diagnostic utility of glypican‐3 for hepatocellular carcinoma on liver needle biopsy. J Clin Pathol. 2010;63:599‐603. [DOI] [PubMed] [Google Scholar]
- 59. Yamauchi N, Watanabe A, Hishinuma M, et al. The Glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol. 2005;18:1591‐1598. [DOI] [PubMed] [Google Scholar]
- 60. Libbrecht L, Severi T, Cassiman D, et al. Glypican‐3 expression distinguishes small hepatocellular carcinoma s from cirrhosis, dysplastic nodules. Am J Surg Pathol. 2006;30:1405‐1411. [DOI] [PubMed] [Google Scholar]
- 61. Sung YK, Hwang SY, Park MK, et al. Glypican‐3 is overexpressed in human hepatocellular carcinoma. Cancer Sci. 2003;94:259‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kawaida M, Yamazaki K, Tsujikawa H, et al. Diffuse and canalicular patterns of glypican‐3 expression reflect malignancy of hepatocellular carcinoma. Pathol Int. 2019;69:125‐134. [DOI] [PubMed] [Google Scholar]
- 63. Fu S‐J, Qi C‐Y, Xiao W‐K, et al. Glypican‐3 is a potential prognostic biomarker for hepatocellular carcinoma after curative resection. Surgery. 2013;154:536‐544. [DOI] [PubMed] [Google Scholar]
- 64. Wang YL, Zhu ZJ, Teng DH, et al. Glypican‐3 expression and its relationship with recurrence of HCC after liver transplantation. World J Gastroenterol. 2012;18:2408‐2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen I‐P, Ariizumi S‐I, Nakano M, et al. Positive glypican‐3 expression in early hepatocellular carcinoma predicts recurrence after hepatectomy. J Gastroenterol. 2014;49:117‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sayem MA, Tomita Y, Yuno A, et al. Identification of glypican‐3‐derived long peptides activating both CD8+ and CD4+ T cells; prolonged overall survival in cancer patients with Th cell response. Oncoimmunology. 2016;5:e1062209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337‐339. [DOI] [PubMed] [Google Scholar]
- 68. Brunsvig PF, Kyte JA, Kersten C, et al. Telomerase peptide vaccination in NSCLC: a phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8‐year update on a phase I/II trial. Clin Cancer Res. 2011;17:6847‐6857. [DOI] [PubMed] [Google Scholar]
- 69. Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8:351‐360. [DOI] [PubMed] [Google Scholar]
- 70. Sawada YU, Yoshikawa T, Shimomura M, et al. Programmed death‐1 blockade enhances the antitumor effects of peptide vaccine‐induced peptide‐specific cytotoxic T lymphocytes. Int J Oncol. 2015;46:28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2


