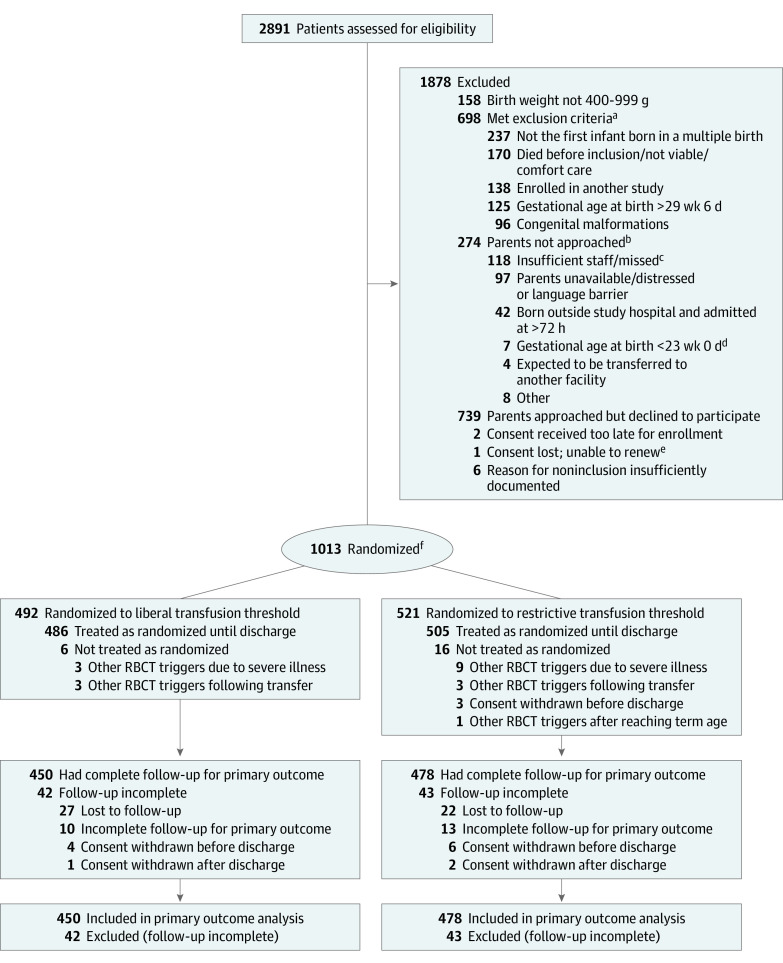
Figure 1. Flow of Participants in the Effects of Transfusion Thresholds on Neurocognitive Outcomes of Extremely Low-Birth-Weight Infants (ETTNO) Trial.
RBCT indicates red blood cell transfusion.
aMore than 1 reason was possible.
bMore than 1 reason was possible. Because investigators’ decisions not to approach parents could have introduced selection bias (eg, by preventing the sickest infants to enter this study), the study population was compared with the cohort of the German Neonatal Network database and no indication of selection bias was found (eTable 3 in Supplement 2).
c“Missed” indicates not approached despite being eligible; reason not known.
dGestational age at birth <23 weeks was not a predefined exclusion criterion, but some centers opted not to include these infants. Ten infants with gestational age at birth <23 weeks are listed under various other exclusion criteria.
eAccording to the investigator at the site, the parents provided consent, but during on-site monitoring (after discharge), no signed consent form was found, and investigators were unable to locate the family to renew the consent.
fRandomization was stratified by center and birth weight stratum (400-749 g and 750-999 g). Stratification by 36 centers and variable block size (2-10) accounted for the difference in the number of enrolled infants between treatment groups.