Abstract
Background
Time to sustained seizure frequency reduction can provide clinically meaningful epilepsy outcomes.
Aims of the Study
To examine the time course of brivaracetam (BRV) efficacy in adults with focal seizures and focal to bilateral tonic‐clonic seizures (FBTCS).
Methods
Post hoc analysis of data pooled from three randomized controlled trials of oral adjunctive BRV in adults with epilepsy. Patients with focal epilepsy and a subpopulation with FBTCS receiving BRV 50, 100, or 200 mg/d (initiated without up‐titration) or placebo for 12 weeks were analyzed for time to sustained ≥75%, ≥90%, and 100% seizure reduction without interruption from first day until trial ends.
Results
Evaluation included 1160 patients with focal seizures, including 352 patients with FBTCS. Sustained ≥75%, ≥90%, and 100% response in focal seizures was higher from day 1 for BRV 100 and 200 mg/d vs placebo (P < .01). Sustained ≥75% and 100% FBTCS reduction from day 1 was higher for BRV 100 and 200‐mg/d groups vs placebo (P < .01).
Conclusions
The majority of patients achieving 75%‐100% sustained seizure frequency reduction (all focal seizure types and the subpopulation with FBTCS) with oral BRV (100 or 200 mg/d) achieved this response on the first‐treatment day.
Keywords: antiepileptic drugs, focal epilepsy, focal to bilateral tonic‐clonic seizures, partial‐onset seizures, sustained seizure reduction
1. INTRODUCTION
A common primary measure of antiepileptic drug (AED) efficacy is the 50% responder rate (RR), reflecting the proportion of patients with ≥50% seizure frequency reduction during a given treatment period. However, RR does not capture seizure frequency distribution, the timing, or persistence of the seizure reduction response, and includes patients who discontinue treatment.
We previously proposed the concept of “sustained ≥50% response,” 1 which is a response that is maintained without interruption or treatment discontinuation from the day it is first achieved and continues for the remainder of the treatment period. It allows assessment of the time to onset of sustained ≥50% responder status (SRS), indicates duration of AED treatment required to achieve ≥50% SRS, and excludes patients who subsequently lose that response or discontinue the trial. 1
In our earlier post hoc analysis of pooled data from three pivotal randomized controlled trials of oral adjunctive brivaracetam (BRV) treatment of focal (partial‐onset) seizures, BRV‐treated patients had a higher sustained ≥50% response rate (SRR) vs placebo‐treated patients starting on the first day of treatment (15.5%, 18.1%, and 19.4% for BRV 50, 100, and 200 mg/d, respectively, vs 6.7% for placebo). 1 For comparison, overall ≥50% RR was 34.2%, 39.5%, and 37.8% for respective BRV doses vs 20.3% for placebo. Although ≥50% seizure frequency reduction is accepted as a regulatory outcome, its clinical usefulness has been questioned 1 ; ≥75%, ≥90%, and 100% seizure frequency reduction may be more clinically meaningful. We therefore evaluated the same pooled data set to determine time to reach sustained ≥75%, ≥90%, and 100% response. Because focal to bilateral tonic‐clonic seizures (FBTCS) have serious ramifications (eg, falls, injury, mortality), we also evaluated time to sustained ≥75%, ≥90%, and 100% FBTCS frequency reduction.
2. METHODS
Efficacy data were pooled from phase 3 clinical trials (N01252 [NCT00490035], 2 N01253 [NCT00464269], 3 N01358 [NCT01261325] 4 ) enrolling adults (≥16 years) with focal seizures. All three initial clinical trials received approval by appropriate institutional review committees and were conducted in accordance with all regulatory, ethical, and good clinical practice requirements. After an 8‐week prospective Baseline period, patients were randomized to BRV or placebo without titration and entered a 12‐week treatment period. 1 Patients randomized to BRV 50‐200 mg/d or placebo were included in the analysis. Similar to our earlier report, because patients taking concomitant LEV were excluded from the N01358 trial, patients from N01252 and N01253 who also were taking concomitant levetiracetam were excluded from this analysis. 1 Time points included treatment days 1‐84.
Because the statistical method estimates the probability of a treatment effect over time, efficacy outcomes included Kaplan‐Meier (KM) estimates of time to ≥75%, ≥90%, and 100% SRS based on focal seizure frequency at Baseline for the overall population and FBTCS frequency at Baseline for the FBTCS subpopulation (subset of patients reporting FBTCS during baseline). SRR was defined as the estimated percentage of patients in the overall population and FBTCS subpopulation who attained a ≥75%, ≥90%, or 100% reduction in seizure frequency from baseline from the day this was first achieved and continued (was sustained) without interruption for every successive day through day 84. Given this definition, patients who discontinued treatment during the maintenance period were not classified as achieving a sustained response. Percentages of patients attaining sustained response by number of all prior AEDs also were explored. The ≥75%, ≥90%, and 100% RRs were defined similarly to ≥50% RRs.
Incidences of treatment‐emergent adverse events (TEAEs) were assessed weekly and are reported for the safety populations (overall and FBTCS subpopulation). 5 Discontinuations due to TEAEs have been reported for the overall population. 1
Kaplan‐Meier estimates of the proportion of patients achieving ≥75%, ≥90%, and 100% SRS, and P‐values for treatment differences (vs placebo) were calculated, similar to our earlier report. 1 To control for multiplicity, the statistical significance of each BRV treatment group vs placebo was assessed starting on the last day of the maintenance period (day 84) and stepping backward for each day until P ≤ .05 for all days or P > .05 for any day from day 84 to day 1. The P‐values were adjusted for multiple testing for each BRV dose arm vs placebo using the gatekeeping strategy suggested by Dmitrienko et al 6 to preserve the overall false‐positive (ie, Type I) error rate. The statistical comparisons of each BRV dose arm to placebo were carried out at the .05 significance level. With the gatekeeping procedure, the treatment arms for each day were tested in a sequential manner during the treatment period and the tests for each subsequent day were performed only if the tests for the previous treatment arm in that day were significant. Starting with day 84, statistical significance was assessed for each BRV dose arm versus placebo. If statistical significance was achieved at day 84, then statistical significance was assessed at day 83 for each BRV dose arm versus placebo. If statistical significance was achieved at day 83, statistical significance was assessed at day 82 for each BRV dose arm versus placebo. This testing strategy continued for days 81, 80, and so forth down to either day 1 or until a day was reached for which statistical significance was not achieved for at least one BRV dose arm. Time to achieving a sustained responder status was then considered to have been statistically demonstrated for the lowest day for which statistical significance was achieved for BRV dose arms for all days from that day through day 84. P‐values were assessed using a log‐rank test and are nominal.
3. RESULTS
3.1. Patient population
The efficacy population comprised 1160 patients (BRV 50‐200 mg/d, n = 742; placebo = 418), including 352 patients with FBTCS (BRV, n = 237; placebo = 115). Baseline characteristics of the pooled population with focal seizures 1 , 5 and the FBTCS subpopulation 7 have been previously reported.
3.2. Efficacy
For the overall population, ≥75% SRRs on day 1 were 10.8% and 9.3% for BRV 100 and 200 mg/d vs 3.1% placebo (P < .01), and increased across BRV doses and placebo through day 84 (19.6%, 17.8% vs 6.7%, respectively; P < .01; Figure 1A). Overall, KM estimates of achieving a ≥75% response on day 1 were 79.8% and 81.0% for patients treated with BRV 100, or 200 mg/d, respectively, vs 65.6% for placebo‐treated patients (P < .0001 for each dose vs placebo).
FIGURE 1.
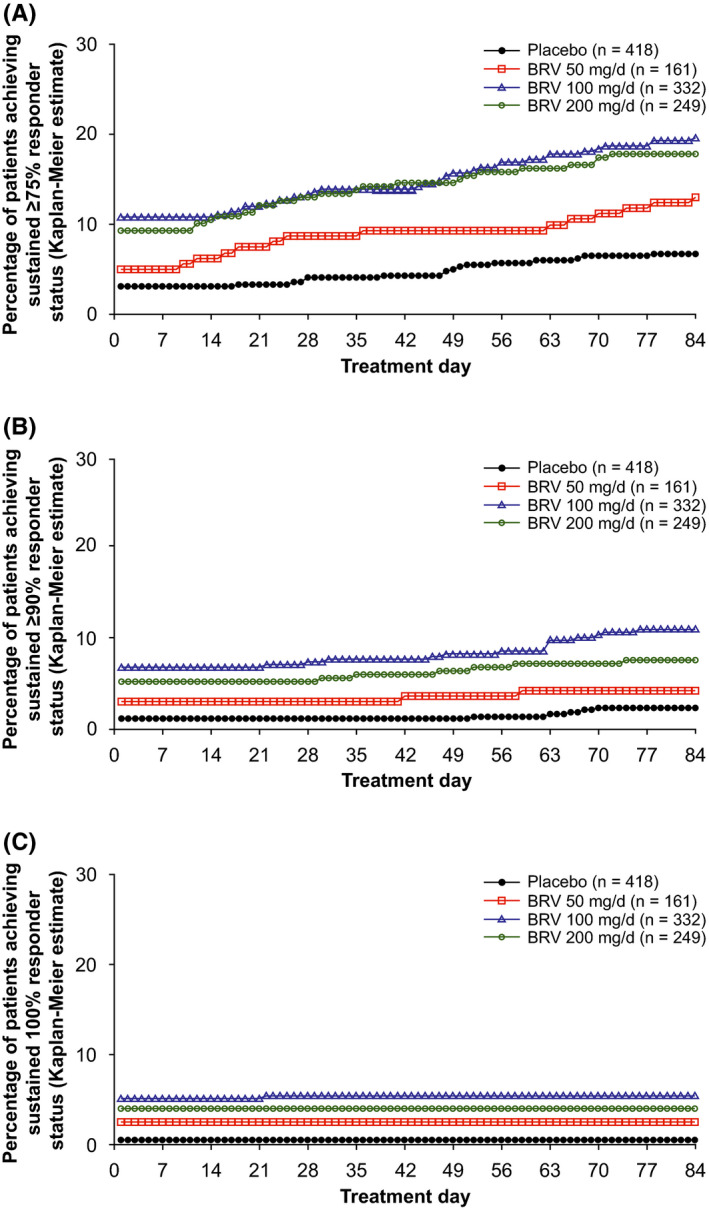
Kaplan‐Meier estimates of time to onset of sustained response in patients with focal seizures from treatment day 1 through treatment day 84. A, Sustained ≥75% responder status. For each day, P < .01 for BRV 100 and 200 mg/d vs placebo; P < .05 at days 16‐48 and 74‐84 for the BRV 50‐mg/d group. B, Sustained ≥90% responder status. For each day, P < .01 for BRV 100 and 200 mg/d vs placebo; P < .05 at days 42‐51 and 59‐62 for the BRV 50‐mg/d group. C, Sustained 100% responder status. For each day, P < .05 for BRV 50 mg/d, and P < .01 for BRV 100 and 200 mg/d, vs placebo. Analyses performed on the efficacy population. 5 BRV, brivaracetam
The ≥90% SRRs on day 1 were 6.9% and 5.3% for BRV 100 and 200 mg/d vs 1.2% placebo (P < .01) and increased to 11.1% and 7.7% vs 2.4% at day 84 (P < .01; Figure 1B). Overall, KM analysis estimated that 78.9%, 81.0%, and 65.1% of all patients treated with BRV 100 and 200 mg/d, and placebo achieved a 90% response on treatment day 1 (P < .0001 for each dose vs placebo) (Table S1). The ≥75% and ≥90% SRRs trended non‐significantly higher for the BRV 50‐mg/d group vs placebo (Figure 1A,B; Table S1). The 100% SRRs on day 1 were 5.1% and 4.0% for BRV 100 and 200 mg/d, vs 0.5% for placebo (P < .05; Figure 1C), and remained essentially the same by day 84.
The KM estimates for patients with sustained ≥75% response with BRV 100 or 200 mg/d were similar across groups with two or fewer, three to four, and five to six prior AEDs; sustained ≥90% response was similar for two or fewer and three to four prior AEDs (Figure 2). Patients with prior exposure to seven or more AEDs had lesser response.
FIGURE 2.
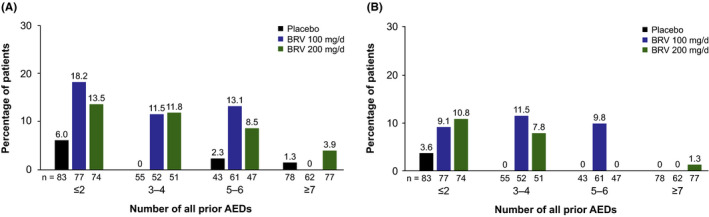
Percentage of patients attaining sustained response (at treatment day 1) by number of all prior AEDs. A, Sustained ≥75% response and B, sustained ≥90% response. In each trial, prior AEDs were discontinued before study entry. N01358 was the only trial capturing all history of prior AEDs used before entry; N01252 and N01253 collected AED use within 5 y before study entry and were not included. Analyses performed on efficacy population. 5 AED, antiepileptic drug; BRV, brivaracetam
In the FBTCS subpopulation, the ≥75%, ≥90%, and 100% SRRs were higher from day 1 for BRV 100 and 200 mg/d vs placebo (Figure 3A‐C; Table S2): respectively, 35.0% and 41.3% vs 15.7% for ≥75% SRR; 34.0%, 37.3% vs 14.8% for ≥90% SRR; and 32.0%, 36.0% vs 14.8% for 100% SRR (all P < .01). Respective SRRs through day 84 were 45.0%, 45.3% vs 20.9% for ≥75% SRR; 36.0%, 40.0% vs 15.7% for 90% SRR, and unchanged from day 1 for 100% SRR (all P < .01). The BRV 50‐mg/d group had non‐significantly higher SRRs vs placebo on day 1 (Table S2). The majority of patients achieving ≥75%, ≥90%, and 100% responses did so on treatment day 1 (KM estimated response: 87.8%‐95.2%).
FIGURE 3.
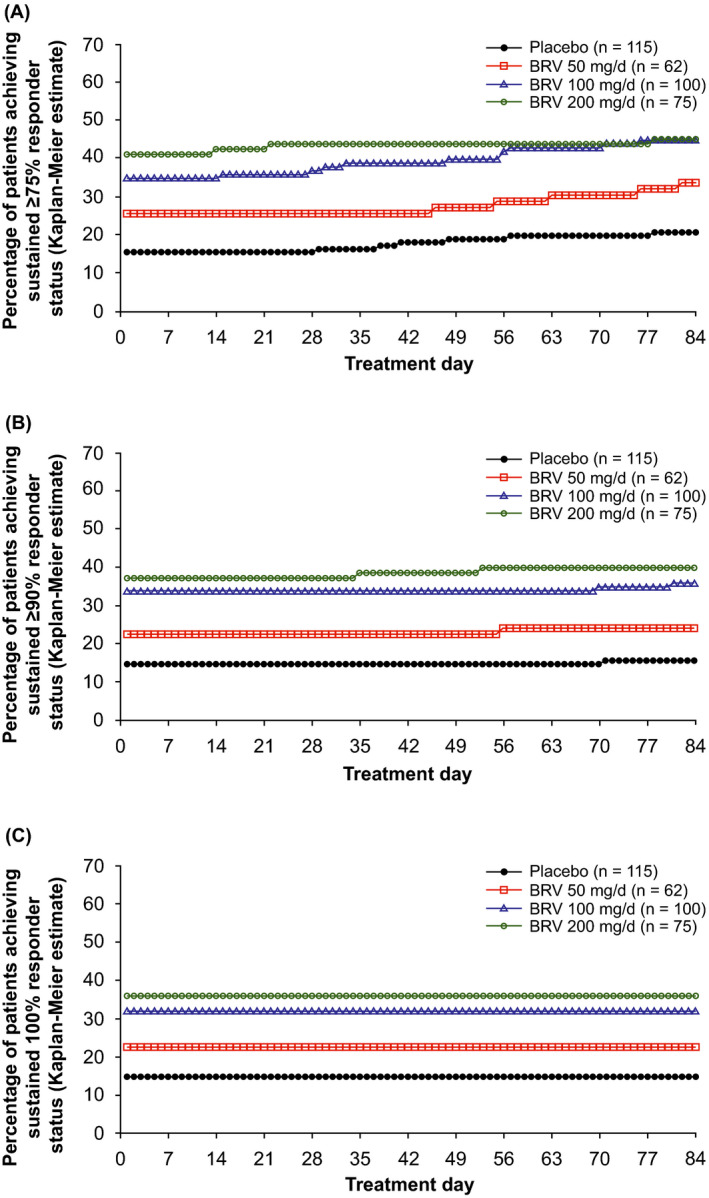
Kaplan‐Meier estimates of time to onset of sustained response in patients with FBTCS from treatment day 1 through treatment day 84. A, Sustained ≥75% responder status. For each day, P < .01 for BRV 100 and 200 mg/d vs placebo; P‐values were not significant (NS) for BRV 50 mg/d vs placebo. B, Sustained ≥90% responder status. For each day, P < .01 for BRV 100 and 200 mg/d vs placebo; P‐values were NS for BRV 50 mg/d vs placebo. C, Sustained 100% responder status. For each day, P < .01 for BRV 100 and 200 mg/d vs placebo; P‐values were NS for BRV 50 mg/d vs placebo. Analyses performed on efficacy population. 5 BRV, brivaracetam; FBTCS, focal to bilateral tonic‐clonic seizures
3.3. Safety
In the overall safety population (N = 1262), the incidence of TEAEs was highest during the first week of treatment (BRV 50 mg/d: 64/200 [32.0%] patients; 100 mg/d: 128/353 [36.3%]; 200 mg/d: 102/250 [40.8%]; placebo: 108/459 [23.5%]) and declined by treatment end. In the FBTCS safety population (n = 381), the incidence of TEAEs peaked during week 1 for both BRV (22/73 [30.1%]; 33/106 [31.1%], and 31/75 [41.3%] for BRV 50, 100, and 200 mg/d) and placebo (26/127 [20.5%]), and decreased thereafter. The incidence of TEAEs leading to discontinuation in the FBTCS subpopulation during week 1 was 0% (0/73), 1.9% (2/106), 1.3% (1/75), and 0.8% (1/127) for BRV 50, 100, and 200 mg/d, and placebo.
4. DISCUSSION
In this post hoc analysis, ≥75%, ≥90%, and 100% SRS in focal seizure reduction was observed with oral adjunctive BRV beginning on day 1, was higher for BRV‐ vs placebo‐treated patients, and increased over time for ≥75% and ≥90% SRS. The majority of patients who achieved ≥75%, ≥90%, and 100% SRS did so on the first day of treatment. These results extend our previous findings 1 by showing that BRV at recommended starting doses results in a sustained seizure reduction from the first day of treatment not only at the ≥50% response level but also at the clinically more meaningful ≥75%, ≥90%, and 100% seizure reduction.
In the most severe focal seizure type, FBTCS, ≥75%, ≥90%, and 100% SRR was observed from the first day of treatment. Over one‐third of patients with FBTCS treated with BRV 100 and 200 mg/d achieved FBTCS seizure freedom on the first‐treatment day and sustained it for the entire treatment period. The high SRR from the first day of treatment may be particularly important for patients with FBTCS, who are at high risk of falls, injury, and mortality, including sudden unexpected death in epilepsy. 8
The results observed in our current analysis for BRV are consistent with a similar study by Naritoku et al 9 for lamotrigine extended release (LTG). Using a slightly different definition for time to ≥50% reduction in seizure frequency (defined as the time a patient achieved and maintained a ≥50% reduction in seizure frequency after ≥1 week of the double‐blind treatment period), Naritoku et al 9 reported the time to ≥50% reduction was shorter for patients treated with LTG vs placebo (P = .0007). This treatment difference reached statistical significance after 18 days of the dose‐escalation phase of the trial. A major difference between the Naritoku et al trial and our analysis is that a dose‐escalation, or titration, phase was not included for patients in the BRV trials used in our pooled analysis.
The sustained response observed in our current report, as well as those from our previous analysis of patients achieving ≥50% SRS on day 1, 1 further supports the suggestion that the effectiveness of BRV occurs early in clinical treatment. One reason for this early onset of efficacy may be due to the lack of the need for titration because of BRV's established favorable safety profile. 1 , 5 Pharmacokinetic analysis in healthy volunteers indicated that the absorption of oral BRV reaches a median t max at 0.5‐1 hour with a time to peak plasma concentrations (C max) at 0.5‐3.5 hours. 10 Studies in healthy volunteers also suggest that intravenous infusion of 200‐mg BRV enters the brain and binds to the SV2A protein (its target protein) within corrected half‐time values of 1.7 to 2.1 minutes compared to 20.5 (±5.7) minutes for LEV 1500 mg. 11 Therefore, the rapid adsorption of BRV may help to explain, at least in part, the observed efficacy of BRV in patients with epilepsy starting at day 1 and without the need for titration.
Interpretation of our findings is limited by the nature of post hoc analyses, pooling of clinical trial data, and minor between‐trial differences in trial populations. A potentially noteworthy limitation is the relatively short treatment duration used in the analysis. The results from our analysis indicate the SRRs increased across all three BRV dose levels from day 1 through day 84, which is approximately 3 months. However, observations from Brodie et al 12 suggest that up to 22% of patients newly diagnosed with epilepsy starting a specific AED treatment may achieve and sustain seizure freedom after 6 months or longer. In addition, a retrospective, database analysis by Faught et al 13 reported that 39% of patients receiving first‐line monotherapy for epilepsy needed changes their AED treatment regimen within 1year. Finally, Cassard et al 14 years. Therefore, the short duration of the data in this study may not have captured those patients who achieved a sustained response after 3 months of treatment or those patients who no longer had a sustained response beyond the 84‐day cutoff. Additional investigation using long‐term clinical trial data would likely address these limitations. 12 , 13
In conclusion, this analysis shows that a majority of patients with focal seizures who achieve 75%‐100% seizure frequency reduction with BRV do so on the first day of treatment.
CONFLICT OF INTEREST
P. Klein has served as a consultant for Abbott, SK Life Science and Neurelis, a speaker for Sunovion, a consultant, advisory board member, and speaker for Aquestive, Eisai, and UCB Pharma, is a member of the Medical Advisory Board of Alliance and of the Scientific Advisory Board of OB Pharma, and has received research support from Lundbeck, and from CURE/Department of Defense. C. Laloyaux, S. Elmoufti, T. Gasalla, and M. S. Martin are employees of UCB Pharma.
AUTHOR CONTRIBUTIONS
Pavel Klein, MB BChir and Cédric Laloyaux, PhD were involved in the design and conceptualization of the study, the analysis and interpretation of data, drafting and revising the manuscript for intellectual content, and final approval of the manuscript. Sami Elmoufti, MSc was involved in the design and conceptualization of study, acquisition of data, statistical analyses, analysis and interpretation of data, drafting and revising the manuscript for intellectual content, and final approval of the manuscript. Teresa Gasalla, MD was involved in the analysis and interpretation of data, drafting and revising the manuscript for intellectual content, and final approval of the manuscript. Melinda S. Martin, PhD was involved in the design and conceptualization of the study, analysis and interpretation of data, drafting and revising the manuscript for intellectual content, and final approval of the manuscript.
Supporting information
Table S1‐S2
ACKNOWLEDGMENTS
The authors thank the patients and their caregivers, in addition to the investigators and their teams who contributed to these trials. The authors acknowledge Helen Ysak, PharmD (UCB Pharma, Smyrna, GA) for overseeing publication development and Svetlana Dimova, MD, PhD (UCB Pharma, Brussels, Belgium) for support with data analysis and interpretation. Lynne Isbell, PhD, CMPP and Richard Fay, PhD, CMPP (Evidence Scientific Solutions, Philadelphia, PA, USA) provided writing assistance, which was funded by UCB Pharma.
Klein P, Laloyaux C, Elmoufti S, Gasalla T, Martin MS. Time course of 75%–100% efficacy response of adjunctive brivaracetam. Acta Neurol Scand. 2020;142:175–180. 10.1111/ane.13287
Statistical analysis: These analyses were conducted by S. Elmoufti (UCB Pharma).
Trial registration: N01252: NCT00490035; N01253: NCT00464269; N01358: NCT01261325
Funding information
This study was funded by UCB Pharma.
DATA AVAILABILITY STATEMENT
Data can be made available upon request to qualified researchers (www.clinicalstudydatarequest.com).
REFERENCES
- 1. Klein P, Johnson ME, Schiemann J, Whitesides J. Time to onset of sustained ≥50% responder status in patients with focal (partial‐onset) seizures in three phase III studies of adjunctive brivaracetam treatment. Epilepsia. 2017;58(2):e21‐e25. [DOI] [PubMed] [Google Scholar]
- 2. Ryvlin P, Werhahn KJ, Blaszczyk B, Johnson ME, Lu S. Adjunctive brivaracetam in adults with uncontrolled focal epilepsy: results from a double‐blind, randomized, placebo‐controlled trial. Epilepsia. 2014;55(1):47‐56. [DOI] [PubMed] [Google Scholar]
- 3. Biton V, Berkovic SF, Abou‐Khalil B, Sperling MR, Johnson ME, Lu S. Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: a phase III randomized, double‐blind, placebo‐controlled trial. Epilepsia. 2014;55(1):57‐66. [DOI] [PubMed] [Google Scholar]
- 4. Klein P, Schiemann J, Sperling MR, et al. A randomized, double‐blind, placebo‐controlled, multicenter, parallel‐group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial‐onset seizures. Epilepsia. 2015;56(12):1890‐1898. [DOI] [PubMed] [Google Scholar]
- 5. Ben‐Menachem E, Mameniškienė R, Quarato PP, et al. Efficacy and safety of brivaracetam for partial‐onset seizures in 3 pooled clinical studies. Neurology. 2016;87(3):314‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dmitrienko A, Tamhane AC. Gatekeeping procedures with clinical trial applications. Pharm Stat. 2007;6(3):171‐180. [DOI] [PubMed] [Google Scholar]
- 7. Moseley BD, Sperling MR, Asadi‐Pooya AA, et al. Efficacy, safety, and tolerability of adjunctive brivaracetam for secondarily generalized tonic‐clonic seizures: pooled results from three phase III studies. Epilepsy Res. 2016;127:179‐185. [DOI] [PubMed] [Google Scholar]
- 8. Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol. 2016;15(10):1075‐1088. [DOI] [PubMed] [Google Scholar]
- 9. Naritoku DK, Warnock CR, Messenheimer JA, et al. Lamotrigine extended‐release as adjunctive therapy for partial seizures. Neurology. 2007;69(16):1610‐1618. [DOI] [PubMed] [Google Scholar]
- 10. Sargentini‐Maier ML, Rolan P, Connell J, et al. The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after single increasing oral doses in healthy males. Br J Clin Pharmacol. 2007;63(6):680‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finnema SJ, Rossano S, Naganawa M, et al. A single‐center, open‐label positron emission tomography study to evaluate brivaracetam and levetiracetam synaptic vesicle glycoprotein 2A binding in healthy volunteers. Epilepsia. 2019;60(5):958‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brodie MJ, Barry SJ, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78(20):1548‐1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Faught E, Helmers S, Thurman D, Kim H, Kalilani L. Patient characteristics and treatment patterns in patients with newly diagnosed epilepsy: a US database analysis. Epilepsy Behav. 2018;85:37‐44. [DOI] [PubMed] [Google Scholar]
- 14. Cassard L, Hegde M, Gidal B, et al. Levetiracetam versus sodium channel blockers as first prescribed antiepileptic drug: data from the human epilepsy project. AES 2019 Annual Meeting Abstract Database AESnetorg. 2019;Abst: 1.318. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2
Data Availability Statement
Data can be made available upon request to qualified researchers (www.clinicalstudydatarequest.com).


