Figure 3.
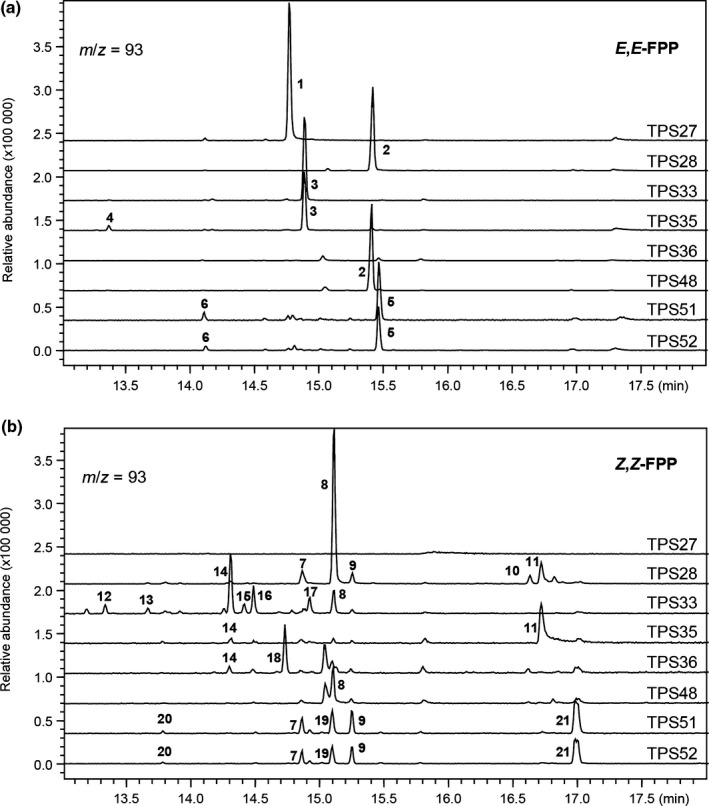
GC–MS analysis of the products formed in vitro by the enzymatic activities of tomato TPS proteins. Enzymes were incubated with E,E‐FPP (a) and Z,Z‐FPP (b) and products were analysed as described in the ‘Materials and Methods’ section. Reaction products were identified by comparison of their mass spectra and retention indices with authentic standards and NIST libraries: 1, α‐farnesene; 2, elemol; 3, guaia‐1(10),11‐diene; 4, β‐elemene; 5, E‐nerolidol; 6, E‐β‐farnesene; 7, E‐β‐bisabolene; 8, unknown 1; 9, α‐bisabolene; 10, α‐eudesmol; 11, (Z,Z)‐farnesol; 12, α‐cedrene; 13, cis‐α‐bergamotene; 14, β‐acoradiene; 15, unknown 2; 16, unknown 3; 17, β‐curcumene; 18, cis‐muurola‐3,5‐diene; 19, Z‐nerolidol; 20, Z‐β‐farnesene; 21, α‐bisabolol. Mass spectra and retention indices of the terpene products can be found in Supporting Information Fig. S3 and Table S2, respectively. FPP, farnesyl diphosphate; TPS, terpene synthase.
