Abstract
HBoV1 is small DNA virus that belongs to the Bocaparvovirus genus of the Parvoviridae family. HBoV1 is a common respiratory pathogen causing mild to life-threatening acute respiratory tract infections in children and immunocompromised individuals. HBoV1 infects both the upper and lower respiratory tracts. HBoV1 infection causes death of airway epithelial cells, resulting in airway injury and inflammation. In vitro, HBoV1 only infects well-differentiated (polarized) human airway epithelium (HAE) cultured at an air-liquid interface (HAE-ALI), but not any dividing human cells. A full-length HBoV1 genome of 5,543 nucleotides has been cloned from DNA extracted from a human nasopharyngeal swab into a plasmid called HBoV1 infectious clone pIHBoV1. Transfection of pIHBoV1 replicates efficiently in human embryonic kidney (HEK)293 cells and produces virions that are highly infectious. This chapter describes the protocols for the production of HBoV1 in HEK293 cells, the generation of HAE-ALI cultures, and the infection of HBoV1 in HAE-ALI.
Introduction
Human bocavirus 1 (HBoV1) is an autonomously replicating human parvovirus belonging to the species Primate bocaparvovirus 1 in genus Bocaparvovirus; family Parvoviridae (Cotmore, Agbandje-McKenna et al., 2019). HBoV1 infection causes acute respiratory tract infections (ARTI) in young children (Qiu, Söderlund-Venermo et al., 2017;Christensen, Kesti et al., 2019) with symptoms manifested as mild symptoms (similar to common cold) or severe pneumonia and bronchiolitis (Choi, Lee et al., 2006;Allander, Jartti et al., 2007;Schildgen, Muller et al., 2008;Arnold, Singh et al., 2006;Schildgen, 2010;Manning, Russell et al., 2006;Kesebir, Vazquez et al., 2006;Fry, Lu et al., 2007;Wang, Wang et al., 2010;Don, Söderlund-Venermo et al., 2011;Blessing, Neske et al., 2009;Don, Söderlund-Venermo et al., 2010;Ursic, Jevsnik et al., 2012;Ursic, Steyer et al., 2011;Nascimento-Carvalho, Cardoso et al., 2012;Terrosi, Fabbiani et al., 2007;Calvo, Garcia-Garcia et al., 2008;Longtin, Bastien et al., 2008;Pozo, Garcia-Garcia et al., 2007;Lu, Chittaganpitch et al., 2006;Neske, Blessing et al., 2007;Moriyama, Hamada et al., 2010;Ruohola, Waris et al., 2009;Del Rosal, Garcia-Garcia et al., 2015;do Amaral de, Amantea et al., 2013;Rezes, Soderlund-Venermo et al., 2009;Xu, Arku et al., 2017;Schlaberg, Queen et al., 2017). Sometimes, HBoV1 infection can be life-threatening (Ursic, Steyer et al., 2011;Korner, Soderlund-Venermo et al., 2011;Edner, Castillo-Rodas et al., 2011;Jula, Waris et al., 2013;Ursic, Krivec et al., 2015;Tabatabai, Fakhiri et al., 2019). It has been reported that mono-detection of HBoV1 infection is significantly associated with community-acquired pneumonia (Schlaberg, Queen et al., 2017). Primary acute HBoV1 infection can be diagnosed only when high virus loads (>104 viral genome copies per ml of nasopharyngeal aspirate) or viral mRNA are detected in respiratory secretions, or when the patient has HBoV1-specific IgM antibody and/or has an increased HBoV1-specific IgG antibody, or has a viremia (detection of viral DNA in plasma) (Allander, Jartti et al., 2007;Proenca-Modena, Gagliardi et al., 2011;Kantola, Hedman et al., 2008;Wang, Wang et al., 2010;Christensen, Nordbø et al., 2010;Söderlund-Venermo, Lahtinen et al., 2009;Karalar, Lindner et al., 2010;Lindner, Karalar et al., 2008;Zhao, Yu et al., 2013;Bruning, Susi.P. et al., 2016;Christensen, Døllner et al., 2013;Xu, Arku et al., 2017;Schlaberg, Queen et al., 2017).
In vitro, HBoV1 only infects well-differentiated (polarized) human airway epithelium (HAE) cultured at an air-liquid interface (HAE-ALI) (Dijkman, Koekkoek et al., 2009;Huang, Deng et al., 2012;Deng, Yan et al., 2013;Deng, Li et al., 2014;Shen, Deng et al., 2015;Deng, Yan et al., 2016;Shen, Deng et al., 2016;Wang, Shen et al., 2017;Deng, Zou et al., 2017). The epithelial cells in HAE-ALI are well-differentiated and nondividing, they are permissive to HBoV1 infection and support viral DNA replication (Deng, Yan et al., 2016). Based on all available reports, HBoV1 does not infect any dividing cells or proliferating cells (Huang, Deng et al., 2012;Deng, Yan et al., 2016), in contrast to other autonomously replicating parvoviruses (Cotmore & Tattersall, 2013;Cotmore & Tattersall, 2014;Ganaie & Qiu, 2018). However, HBoV1 proviral DNA replicates in human embryonic kidney (HEK)293 cells, following a typical parvoviral rolling hairpin replication model (Cotmore & Tattersall, 2005;Cotmore & Tattersall, 2014), and produces virions that are highly infectious to HAE-ALI (Huang, Deng et al., 2012). HBoV1 infection in HAE-ALI generates infectious progenies, induces a DNA damage response (DDR), with activation of all three PI3 kinase-like kinases (PI3KKs), ataxia telangiectasia mutated (ATM), ataxia telangiectasia and Rad3-related protein (ATR), and DNA-dependent protein kinase, which are essential for HBoV1 DNA replication (Deng, Yan et al., 2016;Deng, Xu et al., 2016). Two Y-family DNA repair DNA polymerases, Pol η and Pol κ, play an important role in HBoV1 genome replication (Deng, Yan et al., 2016;Deng, Xu et al., 2016). Therefore, HBoV1 DNA replication follows a cellular DNA repair process involving high fidelity Y-family DNA repair DNA polymerases. HBoV1 infection of HAE-ALI results in pyroptosis, a type of cell death that is mediated by the inflammasome (Deng, Zou et al., 2017).
Here, we introduce the practical protocols to produce infectious HBoV1 virions in HEK293 cells to generate polarized HAE-ALI cultures that support HBoV1 production infection. HEA-ALI can be differentiated from primary human airway epithelial cells and proliferating human airway basal stem cells, as well as an immortalized human airway cell line, CuFi-8, which retains the potential to differentiate into airway mucosa. In addition, the methods to infect HAE-ALI and to analyze the infected HAE-ALI, as well as transduction of lentiviral vector, are included.
Basic Protocol 1. HBoV1 Production in HEK293 Cells
pIHBoV1 is an HBoV1 proviral plasmid clone. It harbors a full-length HBoV1 genome of 5.543 kb (GenBank accession no.: JQ923422) with the intact left and right hairpin structures at both termini, which contain all the necessary motifs responsible for viral genome replication and virion assembly (Huang, Deng et al., 2012). HEK293 cells are not permissive to HBoV1 infection; however, the cells support the replication of the HBoV1 genome. The transfection of pIHBoV1 in HEK293 cells can produce HBoV1 infectious virions. At 48 h post-infection, most viruses are retained in the nuclei. Freeze-thaw cycles will lyse the cells and release the viruses to the lysates, in which HBoV1 virions can be concentrated and purified through ultracentrifugation on a CsCl gradient.
Materials
HEK293 cells (#CRL-1573, ATCC; Manassas, VA)
LipoD293 (#SL100668, SignaGen, Gaithersburg, MD)
pIHBoV1 (a full-length clone of HBoV1 (Huang, Deng et al., 2012); available upon request from Dr. Qiu)
Phosphate buffered saline, pH7.4 (PBS)
Piston Gradient Fractionator (BioComp, Fredericton, NB, Canada)
Reichert Full-Range Digital Refractometer (Reichert, Mfr # 13950000)
Ultracentrifuge (Sorvall WX, or Backman Optima XE)
TH641 or Backman SW41 rotor
11-ml ultracentrifuge tube
- Slide-A-Lyzer™ cassette (0.5–3 ml, 10,000 or 20,000 MVCO. Thermo Scientific, Rockford, IL)
- Culture HEK293 cells on five 150-mm plates to 80% confluence. For each 150-mm plate, transfect cells with 15 μg of pIHBoV1 using LipoD293 (see Support Protocol 1).
- After incubation for 48 h at 5% CO2 and 37°C, resuspend the cells in 10 ml of PBS (2ml/plate).
- Lyse the cells thoroughly by four cycles of freezing in dry ice/ethanol bath (−72°C) and thawing (in a water bath at 37°C).
- Centrifuge the cell lysate at 10,000 rpm for 30 min to discard the cell debris.
-
Purify the virus in a continuous CsCl gradient:In brief, directly add and dissolve 6.15 g CsCl to 10 ml virus-containing supernatant, which makes the final volume to ~11.5 ml. Spin again at 7,500 g to clarify, then load 11 ml of the sample into an 11-ml ultracentrifuge tube, ultracentrifuge at 36,000 rpm for 36 h at 20°C using a Sorvall TH641 rotor or Backman SW41.
- Collect 22 fractions of 500 μl each with a Piston Gradient Fractionator (BioComp, Fredericton, NB, Canada).
- Determine the density of each fraction by reading the refractive index with a Reichert Digital Refractometer.
- Extract viral DNA from each fraction and quantify with respect to the number of HBoV1 viral genome copies (vgc) using HBoV1-specific quantitative (q)PCR (see Support Protocol 2).
- Dialyze those fractions containing the highest numbers of HBoV1 vgc in a Slide-A-Lyzer™ cassette against PBS and quantify by qPCR for vgc.
Support Protocol 1. HEK293 Cell Culture and Transfection
HEL293 cells (ATCC® CRL-1573) are the only cell line cells that were so far identified to support HBoV1 replication of double HBoV1 genome and produce progeny virions.
Materials
150-mm tissue culture plates
Dulbecco Modified Eagle’s Medium (DMEM) /High glucose with L-glutamine; without sodium pyruvate (HyClone #SH30022; GE Healthcare, Life Sciences)
Fetal bovine serum (FBS; #F0926, Sigma)
Penicillin-Streptomycin (each 5,000 U/ml)
- LipoD293 (#SL100668, SignaGen)
- Culture five 150-mm plates of HEK293 cells in DMEM with 10% FBS and 100 U/ml of Penicillin-Streptomycin (Seed ~4 × 106 cells per plate and take ~2 days to reach a confluency of ~80%).
- Perform transfection when cells are confluent at ~80% (approx. ~2 × 107 cells/plate):
- For each plate, add 15 μg of pIHBoV1 in 750 μl of DMEM (without FBS and Penicillin-Streptomycin) and dilute 45 μl of LipoD293 in 750 μl of DMEM.
- Add diluted LipD293 into the DNA solution and mix well by pipetting up and down for 3–5 times.
- After incubation for 10 min at room template, add the mixture of 1.5 ml drop-wise to the media of the plates (without changing media).
- Incubate transfected cells at 37°C and 5% CO2 for two days.
Support Protocol 2. Quantification of HBoV1 Using Real Time Quantitative PCR
In order to prevent the contamination of viral DNA in the preparation of purified virions, the virions are treated with nuclease before extraction of viral genome, and therefore, the copies of DNAase (Benzonase® Nuclease) digestion resistant particles are determined.
Materials
CsCl fraction with virus collected in Basic Protocol 1
Benzonase® Nuclease (#E1014, Sigma, St Louis, MO)
Proteinase K (15 mg/ml)
QIAamp DNA Blood Mini Kit (#51106, Qiagen)
Applied Biosystems 7500 Fast system (Foster City, CA)
Forward primers, 5’-GCA CAG CCA CGT GAC GAA-3’ (nt 2,391 to 2,408);
Reverse primer, 5’-TGG ACT CCC TTT TCT TTT GTA GGA-3’ (nt 2,466 to 2,443)
TaqMan® probe, 5’−6FAM-TGA GCT CAG GGA ATA TGA AAG ACA AGC ATC G-3’ Iowa Black FQ (nt 2411 to 2,441).
TaqMan Super Mix (Probe MasterMix; Applied Biological Materials Inc., Vancouver, Canada) Or Premix ExTaq (Takara Bio USA, Madison, WI)
- 7500 Fast Real-Time PCR system (Applied Biosystems®)
- Incubate 100 μl of the sample (e.g. 1:10 dilution of the CsCl fraction) with 25 units of Benzonase® Nuclease for 2 h at 37°C, and then add 20 μl of proteinase K (15 mg/ml).
- Extract viral DNA following the Spin Protocol using the QIAamp DNA blood mini kit and elute in 100 μl of deionized (d)H2O.
-
Set up qPCR reactions.an example of one reaction:DNA sample (2.5 μl)Forward primer (900 nM)Reverse primer (900 nM)TaqMan probe (250 nM)2 × Premix ExTaq PCR mixture (12.5 μl)Add dH2O to 25 μlNote: Primers and probe are kept as stock solutions at 100 μM at −20°C.
- Carry out standard cycle to amplify the extracted viral DNA, together with serially diluted pIHBoV1 plasmid (1 μg/μl) from 1 × 10 −1 to 1 × 10 −9, on 7500 Fast Real-Time PCR system.
- Use the calculation of 1 μg = 1.09 × 1011 viral genome copies (vgc) to establish a standard curve for absolute quantification to obtain the vgc.
Basic Protocol 2. Differentiation of Human Airway Cells at an Air-Liquid Interface
Submerged culture of primary human tracheobronchial epithelial cells freshly isolated from airway tissue, expanded airway basal cells or CuFi-8 cells are possible. However, cells cultured in such a condition won’t undergo mucociliary differentiation. When they are grown on permeable porous supports at an ALI, they differentiate and recapitulate the pseudostratified mucociliary phenotype observed in vivo. After seeding onto the permeable membrane of a Transwell® or MillCell® insert, it is initiated as a submerged culture using expansion medium and supplied to both apical and basolateral chambers. Once the cells get confluent, they are subjected to ‘air-lift’, with the differentiation medium that is supplied only to the basal chamber. After “air-lift”, the basal surface of the cells is in contact with the liquid culture medium, whereas the apical surface is exposed to the air. This configuration mimics the conditions found in the human airways and drives cell differentiation towards a mucociliary phenotype. The complex process of airway epithelial differentiation involves cell–matrix and cell–cell interactions, differentiation of mucous and goblet cells, and acquisition of characteristic epithelial ion transport properties.
Materials
PneumaCult-ALI medium (#05001, StemCell), 500ml
PneumaCult-ALI base medium, 450 ml (#05002)
PneumaCult-ALI 10x Supp, 50 ml, (#05003)
PneumaCult-ALI M-Supp, (#05006), 5 × 1 ml
Hydrocortisone solution (96 μg/ml, 200 ×; #07925, StemCell)
Heparin solution (2 mg/ml, 500 ×; # 07980, StemCell)
Human airway cells
Primary human tracheal and/or bronchial epithelial cells (see Support Protocol 4)
Expanded human airway basal cell culture (see Support Protocol 4)
CuFi-8 cell line (see Support Protocol 3)
Transwell® culture inserts (#3413, Corning): 0.4 μm pore polycarbonate membrane, 6.5 mm in diameter, 0.33 cm2, translucent; collagen IV coating is required (see Support Protocol 5)
Transwell® culture inserts (#3470, Corning): 0.4 μm pore polyester membrane, 6.5 mm in diameter, clear; collagen IV coating is required
Millicell® culture inserts (#PIHP01250, Millipore): 0.4 μm pore polycarbonate membrane, 12 mm in diameter, 0.6 cm2; collagen IV coating is required.
Airway cell expansion medium
BEGM (see Basic Protocol 2) or
- SAGM-H (see Basic Protocol 3)
-
Prepare the Complete ALI Medium:Mix the 450 ml base medium with 50 ml of the 10 × Suppl to 500 ml, then aliquot to 5 × 100 ml. In 100 ml of the mixed medium, add one aliquot of the 1 ml M-supplement (100 ×), 200 μl of Heparin Solution (500 ×), and 500 μl of Hydrocortisone solution (200 ×).Note: Freeze the complete ALI Medium in 50 ml tubes at −80°C for long term use (up to 6 months, and thaw one tube before use.
- Seeding cells (Day 0) onto the inserts for pre-ALI culture is described below. Make sure the cells get a uniform distribution when they are set upon the membrane surface. Add ~400 μl of the ALI medium to the basal chamber. Incubate overnight at 37°C and 5% CO2.
- For primary human tracheal/bronchial epithelial cells, which are dissociated from fresh human airway tissues, the cells are resuspended in BEGM supplemented with 5% FBS. Adjust the cell density to 1.5 × 106 cell/ml, and seed ~1.5 × 105 cells (0.1 ml) into a pre-coated Transwell® insert of 0.33 cm2, or ~3 × 105 cells (0.2 ml) into a pre-coated Millicell® culture insert (0.6 cm2).
- For monolayer cultures of proliferating airway basal cells or CuFi-8 cells, use Accutase to dissociate the cells from the culture dish, as described in Basic Protocol 2, step 3. After spinning and removing the Accutase, resuspend the cell pellet of airway basal cells in SAGM-H medium or CuFi-8 cells in BEGM supplemented with 5% FBS. Count the cells and adjust the cell density to 1.0 × 106 cell/ml, seed ~1 × 105 cells (0.1 ml) into a pre-coated Transwell insert, or ~2 × 105 cells (0.2 ml) into a pre-coated Millicell® culture insert.
- At one day after seeding (Day 1), replace the expansion medium of the apical chamber with ~0.1 or 0.15 ml.
- On the second day after seeding (Day 2), aspirate the media in apical and basal chambers, and change to complete ALI medium (both chambers).
- “Air lift” starts in the morning of Day 3. Carefully aspirate the apical AL medium, and expose the airway cell culture to the air.
- On day 4, aspirate the ALI medium from the basal chamber and feed the cells with 500 μl of the complete ALI medium to the basal chamber ONLY.
- In the first 2 weeks after seeding, change the medium in the basal chamber every 2 days. After the first week, change the medium twice a week.
- In the first week after air-lift, check the culture every day. If medium comes up to the apical chamber, carefully aspirate it from the cell surface.
- Beginning in Week 2 post-airlift, mucus might show up on the apical chamber. If it becomes too heavy, remove the mucus from the apical surface by washing the cells once with 200 μl of D-PBS (without Ca++ and Mg++) at room temperature. This procedure should be repeated as required (approximately once per week) to prevent excessive mucus accumulation.
- It takes 3–4 weeks to polarize and well-differentiate the airway epithelia in vitro. Measure the transepithelial electrical resistance (TEER) with the epithelial Ohm-voltmeter (see Support Protocol 4). The TEER should be greater than 1 kΩ/cm2.
-
Support Protocol 3. Expansion of Human Airway Epithelial Cell Line CuFi-8
CuFi-8 cells are an immortalized human airway epithelial cell line derived from airway epithelial cells of a donor cystic fibrosis patient (genotype CFTR delF580/delF508) (Zabner, Karp et al., 2003). Transformed by a reverse transcriptase component of telomerase, hTERT, and human papillomavirus type16 (HPV-16) E6 and E7 genes, the CuFi-8 cell line can be expanded in collagen-coated plates as the monolayer culture. When grown at an ALI (see Basic Protocol 4), this line is capable of forming polarized differentiated mucociliary epithelium that exhibit transepithelial electrical resistance (TEER) and maintain the ion channel physiology expected for the genotypes.
Materials
CuFi-8 cells (available upon request from Dr. Aloysius Klingelhutz at the University of Iowa)
Antibiotics:
Penicillin-Streptomycin (5,000 U/mL)
Gentamycin (#G1397, Sigma), 50 mg/ml
Amphotericin B (#A9528, Sigma), 1.25 mg in 5 ml of dH2O as stock solution of 0.25 mg/ml
BEGM medium with BulletKIT (#CC-3170, Lonza):
This includes the basal medium and also nine aliquots of growth supplements, including: 1) hydrocortisone, 0.5 ml; 2) bovine pituitary extract, 2 ml; 3) human epidermal growth factor, 0.4 ml; 4) transferrin, 0.5 ml; 5) bovine insulin, 0.5 ml; 6) triiodothronine, 0.5 ml; 7) epinephrine, 0.5 ml; 8) retinoic acid, 0.5 ml and 9) GA-1000 (gentamycin/amphotericin B), 0.5 ml.
Note: When used for the culture of CuFi-8 cells, do not use the vial of GA-1000. The CuFi-8 cells don’t grow well with this Lonza supplement.
Accutase (#07920, StemCell)
Accutase consists of a proprietary mixture of proteolytic and collagenolytic enzymes for use in dissociation of cells from standard and adhesion-coated plasticware. Once thawed, it can be kept at 4°C for up to 2 months.
Note: Do not thaw and preheat in 37°C water bath. Directly add the cold solution to the culture to dissociate the cells, no additional washes or enzyme inhibitors are required to inactivate the enzymes.
D-PBS (Dulbecco’s phosphate buffered saline, no calcium, no magnesium)
CryoStor CS10 (# 07930, StemCell)
T75 culture flask or 100-mm culture dishes, collagen IV coated (see Support Protocol 3)
- 500 ml filter units (Pore Size: 0.22 μm)
-
Prepare complete BEGM mediumAdd all the supplements except for the vial of GA-1000 provided in the BEGM™ medium with BulletKIT kit to the basal medium. Add the antibiotic gentamycin to the final concentration of 50 μg/ml, amphotericin B to the final concentration of 1.25 μg/ml, and pen-strep to the 50 U/ml final concentration. Filter through the 500-ml filter unit before use.Note: Freeze the complete BGEM in 50 ml tubes at −80°C for long term use (up to 6 months, and thaw one tube before use.
-
Culture the cells on collagen IV coated plates (see Support Protocol 3). Seed ~0.5 × 106 cells per 100-mm dish or T75 flask. When they are grown to 90% confluence, split for expansion as in Step 3.Note: Do not let them become overgrown before passage.
- After briefly washing the cells with D-PBS, add ~5 to 10 ml of cold Accutase to each 100-mm plate, let sit at 37°C for 5 to 10 min allowing the cells to detach.
- Collect the cells and spin down the cell pellet and passage at 1:5 for the next round culture. Count cells if needed.
-
For cryopreservation, aliquot 106 of CuFi-8 cells in 1 ml CryoStor CS10 or 10% DMSO in FBS.Note: It is important to cryofreeze early passage cells so that you have stocks to use in the extended future.
-
Support Protocol 4. Expansion of Human Airway Basal Cells
Human airway epithelial cells can be isolated via pronase dissociated from fresh tracheal and bronchial tissue from a human donor (See Keiser and Engelhardt, Curr Protoc Human Genet, Basic Protocol 1 (Keiser & Engelhardt, 2013)). Nonprofit organizations, such as the National Disease Research Interchange (www.ndriresource.org), facilitate provision of human biomaterials for research. The initial cell suspension harvested from fresh tissue is a mixed population of ciliated, columnar, secretory, intermediates and undifferentiated basal cells. It usually contains some nonepithelial cells as well. Among them, the airway basal cells are multipotent stem cells that serve as progenitor cells for the other types of differentiated epithelial cells. Recently, Mou et al demonstrated that SMAD signaling activity correlated with the mucociliary differentiation in the airway, and dual TGF-β/BMP inhibition prevents spontaneous differentiation in culture (Mou, Vinarsky et al., 2016). They developed a feeder-free culture platform with the use of small molecule-mediated dual SMAD signaling inhibition to overcome the growth arrest and irreversible differentiation encountered in standard culturing of primary cells. With this protocol, primary airway cells isolated from a patient donor of specific genotype or disease condition can be expanded as a virtually limitless supply of airway basal cells for the culture of in vitro airway epithelial models.
Materials
Human primary airway cells
Human airway basal cells are isolated from fresh lung tissues from donors using a protocol reported previously (Keiser & Engelhardt, 2013) or a commercially available resource. Ensure that IRB (Institution Review Board) approval is received for experiments.
Antibiotics:
Penicillin-Streptomycin (5,000 U/mL)
Gentamycin (#G1397, Sigma), 50mg/ml
Amphotericin B (#A9528, Sigma), 0.25 mg/ml
SAGM medium with BulletKIT (#CC-3118, Lonza):
This includes the basal medium and also the SAGM SingleQuots supplement of ten aliquots including: 1) fatty acid free BSA, 5 ml; 2) hydrocortisone, 0.5 ml; 3) bovine pituitary extract, 2 ml; 4) human epidermal growth factor, 0.5 ml; 5) transferrin, 0.5 ml; 6) bovine insulin, 0.5 ml; 7) triiodothronine, 0.5 ml; 8) epinephrine, 0.5 ml; 9) retinoic acid, 0.5ml and 10) GA-100 (gentamycin/amphotericin B), 0.5 ml.
A8301 – TGFβ antagonist (#2939, Tocris), in DMSO as 10 mM stock
DMH-1– BMP4 antagonist (#4126, Tocris), in DMSO as 10 mM stock
CHIR99021– WNT agonist (#4423, Tocris), in DMSO as 10 mM stock
Y27632– ROCK inhibitor (#1254, Tocris, or #ALX-270–333, Ezno Life Science), in DMSO as 10 mM stock
Accutase (#07920, StemCell).
Once thawed, keep at 4° for up to 2 months. Do not prep-heat in a water bath; directly add the cold solution to the culture to dissociate the cells.
D-PBS (Dulbecco’s phosphate buffered saline, no calcium, no magnesium)
CryoStor CS10 (#07930, StemCell)
T75 or 100-mm dishes, collagen IV coated
- 0.22 μM 500 ml filter units
-
Prepare complete SAGM-H medium:Add all the supplements provided in the BulletKIT to the basal medium. Add 50 μl of A8301, DMH-1 and CHIR99021 (10 mM in stocks) to the final concentration of 1 μM. Add 500 μl Y27632 (10 mM in stock) to the final concentration of 10 μM. Add pen-strep to a final concentration of 50 U/ml. Filter through a 500-ml filter unit of 0.22 μm before use.Note: Freeze the complete SAGM in 50 ml tubes at −80°C for long term use (up to 6 months, and thaw one tube before use.
- Seed the primary human airway cells (P0 or P1) on a 100-mm dish (pre-coated with collagen) at the density of~0.5 × 106 cells with 10 ml of the SAGM-H medium.
-
Replace medium every day. Usually it takes 3–4 days, then the cells will get to sub-confluence, reaching ~4–5 × 106 cells (when they reach late passage of P6 or P7, it begins a bit slowly, reaching ~4 × 106 cells for 5 days). Split as 1:8 (early passages) to 1:6 (late passages) for expansion.Note: Do not let them become overgrown with more than 90% confluence.
- After briefly washing the cells with D-PBS, add ~5 ml of cold Accutase (to a 100-mm plate), let sit at 37°C for 5–10 min allowing the cells to detach.
- Collect the cells and spin at 300 g, and then resuspend the cell pellet with SAGM-H for the next round culture. Count cells if needed.
- For cyropreservation, freeze 1 million of the early passage cells (preferred P1 or P2) in 1 ml of CryoStor CS10 or 10% DMSO in FBS.
-
Support Protocol 5. Coating the Plastic Dishes and Permeable Membrane of the Inserts
Primary airway epithelial cells and CuFi-8 cells are anchorage-dependent cells. They will not attach to either a plastic surface or membrane without surface pretreatment. We recommend using the human placental collagen type VI (Sigma) as an attachment substrate.
Materials
Collagen IV (#C7521, Sigma)
Glacial acetic acid
Glass Erlenmeyer flask, 25 mL
T75 culture flasks or 100-mm cell culture dishes
Transwell® culture inserts (#3413, Corning): 0.4 μm pore and 6.5 mm diameter, polycarbonate membrane, translucent, 0.33 cm2.
Transwell® culture inserts (#3470, Corning): 0.4 μM pore and 6.5mm diameter, polyester membrane, clear, 0.33 cm2.
- Millicell® culture inserts (#PIHP01250, Millipore): 0.4 μM pore and 12 mm diameter, polycarbonate membrane, 0.6 cm2.
-
Prepare 10 × Collagen IV solution (600 μg/ml): 5 mg of collagen in 8.3 ml of sterile Milli-Q water in a 25 ml Erlenmeyer glass flask or a glass bottle, add 16.6 μl of glacial acetic acid. Warm at 37°C to dissolve the collagen.Note: Do not use plasticware.
- Dilute the 10 × solution with sterile Milli-Q water H2O. Add 75 ml sterile Milli-Q water to get the working solution to 60 μg/ml. Filter-sterilize with a 0.22 μM membrane disk or cassette. Store in a glass bottle at 4°C for one month.
-
Add the collagen IV solution (60 μg/ml) to the tissue-culture plastic surface and the membrane of the inserts for a minimum of 18 h or longer at room temperature. The collagen solution can remain on the plastic surface at room temperature for several days and stay stable until the day of seeding.Note: Use 150 μl of the collagen working solution for a Transwell insert, ~6 ml for a dish of 10-mm, and 12 ml for a dish of 150-mm).
- Remove the liquid collagen from the surface and air-dry.
-
If using the coated inserts/dishes rapidly, rinse the plastic surface at least twice with 1 × PBS to remove all traces of the collagen.Note: Residual liquid collagen is toxic to cells, but it is safe after air-drying completely.
-
Support Protocol 6. Transepithelial Electrical Resistance (TEER) Measurement
Transepithelial electrical resistance (TEER) is a widely accepted quantitative technique to measure the integrity of tight junction dynamics in cell culture models of epithelial monolayer and pseudostratified epithelium cultured on a semipermeable filter insert. TEER value is a strong indicator of the integrity of the cellular barriers. TEER measurement uses chopstick-electrodes placed in the apical and basolateral chambers for the total electrical resistance including the ohmic resistance of the cell layer, the cell culture medium, the semipermeable membrane insert, and the electrode medium interface.
Materials
Millicell® ERS-2 Voltohmmeter with STX01 Electrode (MERS00002, MilliporeSigma)
75% ethanol
- Electrolyte solution (0.15 M NaCl, sterile)
- Immerse the electrode tips in 70% ethanol for 15 min. Allow them to air dry for 15 sec.
- Rinse the electrode in a sterile electrolyte solution.
- Set the MODE switch to Ohms (Ω) and turn the POWER switch On.
- Immerse the electrode in a way that the shorter tip is in the insert and the longer tip is in the outer well. The shorter tip should not contact cells growing on the membrane and the longer tip should just touch the bottom of the outer well. Push the button of “Measure” to read the number.
- Repeat Step 1) and 2) when measuring a second insert.
-
Unit Area Resistance = Resistance (Ω) × Effective Membrane Area (cm2).Note: The membrane area is the area of the insert, for example, 0.33 cm2 for Transwell® culture insert (#3413, Corning).
Basic Protocol 3. HBoV1 Infection in HAE-ALI Cultures
HBoV1 infects HAE-ALI cultures at an efficiency much higher at the apical chamber than at the basolateral chamber (Deng, Yan et al., 2013). HBoV1 infects CuFi-ALI generated from CuFi-8 cells poorer than does primary HAE-ALI generated from primary airway cells (Huang, Deng et al., 2012). Commercially available HAE cultures can also be infected with HBoV1, but the infectivity was lower than the in house made HAE cultures (Deng, Li et al., 2014).
Materials
Polarized ALI cultures
Primary HAE-ALI (in Transwell®, 0.33 cm2, Corning)
Primary HAE-ALI (in Millicell® inserts, 0.6 cm2; Millipore)
CuFi-ALI (in Transwell®, 0.33 cm2, Corning)
Virus: purified from pIHBoV1-infected HEK293 cells (see Basic Protocol 1) or apical washes of infected HAE
- PBS, pH7.4
-
Dilute HBoV1 virus to a desired MOI in PBS as inoculum for infection.Note: If the MOI is 1 vgc/cell, it will take 7–10 days to reach the maximum infection (the peak of apical virus release).
- For ALI cultures on Millicell® inserts (0.6 cm2), apply 150 μl inoculum in the apical chamber. For ALI cultures on Transwell® inserts, (0.33 cm2), use 100 μl.
- Incubate for 2 hours and then aspirate the inoculum from the apical chamber and wash the cells with 200 μl of PBS three times to remove unbound virus.
- Incubate the HAE-ALI culture at 37°C and 5% CO2. Every three days, refresh the media in the basolateral chamber.
- Collect apical washes every two days by incubation of 100 μl PBS for 0.33 cm2 in Transwell® or 200 μl PBS for 0.6 cm2 in Millicell® inserts in the apical chamber for 30 min, and perform qPCR (see Support Protocol 2) to determine virus released as described above.
-
Isolate the cells of the infected ALI cultures (see Support Protocol 7) when the apical washes reach a peak titer.Note: If an MOI of 1 vgc/cell is used for infection of primary HAE-ALI, it will take ~10 days to reach the peak of the virus release (1 × 108 vgc/μl). If an MOI of 1,000 is used, it will only take 3–4 days.
-
Support Protocol 7. Isolation of Infected HAE Cells from the Inserts
Infected HAE can be detached from the insert and directly analyzed by immunofluorescent assay for viral antigen expression or viral genome detection. Here, we introduce a method to isolate the infected cells from the inserts, which can be used for extraction of total RNA, low molecular weight DNA, and protein for analysis.
Materials
Versene (Trypsin-EDTA) solution: 0.25% trypsin in 0.48 mM EDTA.Na4 in PBS, pH7.4.
5 mM EDTA.Na4
- Infected primary HAE-ALI (in Transwell® insert, 0.33 cm2, Corning)
- Warm up 5 mM EDTA, Versene, and PBS (or D-PBS) in the hood at room temperature.
- Wash the Transwell® and the basal chamber with PBS. Add 0.5 ml of Versene to the chamber and add 0.1–0.2 ml of 5 mM EDTA to the Transwell® for several minutes.
- Remove EDTA from the Transwell®, add 0.1–0.2 ml of Versene, and keep the Transwell® plate on a slide warmer for 10 min.
-
Pipet up and down to suspend the cells (to avoid clotting the cells), transfer the isolated cells to 5 ml of DMEM with 5% FBS in a 15-ml tube, and spin them at 300 g for 5 min.Note: These collected cells are ready for extraction of total RNA, low molecular weight DNA, and protein for Northern, Southern and Western blotting analyses, respectively. The cells can also be cytospun onto slides for immunofluorescent assays.
Basic Protocol 4. Transduction of Airway Basal Cells with Lentiviral Vector.
To understand the function of host protein of HAE-ALI in virus replication and infection-induced cellular response, it is important to perform gene knockdown in HAE-ALI. We knocked down the Y family DNA repair DNA polymerase η and κ in HAE-ALI, and identified a critical role of the DNA polymerase η and κ in DNA replication of HBoV1 (Deng, Yan et al., 2016). Polarized HAE is hard to be transduced by lentiviral vectors. Here, we introduce a method (as outlined in Figure 1) to transduce airway epithelial cells shortly after seeding on the insert but before polarization, and obtained a high transduction efficiency, all the cells transduced, and efficient gene knockdown (Deng, Yan et al., 2016;Deng, Zou et al., 2017).
Figure 1. Transfection of HAE-ALI cultures using lentiviral vectors.
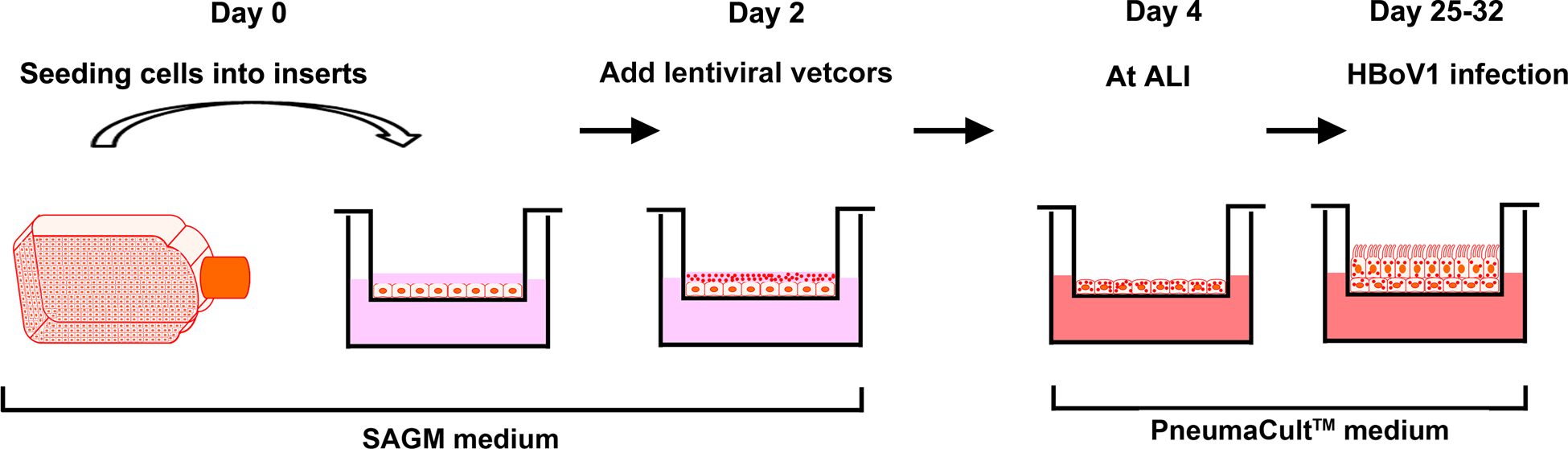
Proliferating human airway epithelial cells (as monolayer) cultured in SAGM-H medium are transferred onto Transwell® inserts. Two days later, the cells are transduced with lentiviruses and cultured in SAGM-H medium for 2 days. On day 4, the medium in the inserts (apical chamber) is removed, and only the basolateral chamber is fed with PneumaCut ALI medium. The cells are further cultured at an ALI for 3 to 4 weeks for fully differentiation before ready for use in virus infection.
Materials
Lentiviral vectors with an mCherry reporter expression cassette.
- Airway epithelial cells in the proliferation stage.
- Use standard methods to produce, purify, and titrate by qPCR the lentiviral vectors such as those describe in Current Protocols (Barde, Salmon et al., 2010).
- Transfer ~1 × 105 of proliferating airway epithelial cells onto each Transwell® insert. After 2 days, infect the cells with a lentiviral vector at an MOI of ~100 vgc/cell.
- After another 2 days, an ALI will be established to induce cell polarization and then PneumaCult-ALI medium will be added to the basolateral chamber.
COMMENTARY
Background Information
An in vitro model of polarized human airway epithelium, which is derived from human airway epithelial cells, is a novel culture system that allows new insights into the infection characteristics of human respiratory viruses (Pyrc, Sims et al., 2010;Banach, Orenstein et al., 2009;Ayora-Talavera, Shelton et al., 2009;Dijkman, Koekkoek et al., 2009;Donaldson, Yount et al., 2008;Scull, Gillim-Ross et al., 2009;Sims, Baric et al., 2005;Zhang, Peeples et al., 2002;Wang, Deering et al., 2000;Jia, Look et al., 2005;Essaidi-Laziosi, Brito et al., 2018;Sheahan, Sims et al., 2020). Isolated airway epithelial cells grown for ~1 month at an air-liquid interface form a pseudostratified mucociliary airway epithelium that displays similar morphologic and phenotypic characteristics to those of in vivo human cartilaginous airway epithelium (Karp, Moninger et al., 2002). Recent studies revealed that the airway epithelium model recapitulates important characteristics of respiratory virus-host cell interactions, such as those seen in the cartilaginous airways of infected lungs (Pyrc, Sims et al., 2010;Sims, Baric et al., 2005;Zhang, Peeples et al., 2002;Palermo, Porotto et al., 2009;Mitchell, Levin et al., 2011;Villenave, Thavagnanam et al., 2012).
In 2009, in vitro HBoV1 infection was reported in well-differentiated HAE (Dijkman, Koekkoek et al., 2009). That study provided valuable information on virus replication. Since 2010, we started using HAE-ALI cultures for HBoV1 infection and studied virus replication and cell death (Huang, Deng et al., 2012;Deng, Yan et al., 2013;Deng, Yan et al., 2016;Deng, Zou et al., 2017). Although variation between donors exists, we usually got better infection in primary HAE-ALI cultures than in CuFi-ALI. The infectivity of HBoV1 in ALI cultures is dependent on the extent of full differentiation of the airway epithelial cells. The higher the TEER can get, the better the HBoV1 infection will be. Studies of HBoV1 infection in HAE-ALI cultures has revealed important characteristics of HBoV1 infection of the airway epithelium (Huang, Deng et al., 2012;Deng, Yan et al., 2013;Deng, Li et al., 2014;Shen, Deng et al., 2015;Deng, Yan et al., 2016;Shen, Deng et al., 2016;Wang, Shen et al., 2017;Wang, Deng et al., 2017;Deng, Zou et al., 2017).
The pHBoV1 is a tool to study the molecular biology of the virus. We have created mutant pIHBoV1 clones that carried mutations at the splice sites, which produced HBoV1 progeny virions, but at a lower level, compared to the wild-type plasmid. Mutant viruses were tested in HAE-ALI, and the results found that a mutant virus that did not express NS3 and NS4 replicated in HAE-ALI as effectively as the wild-type virus; however, the mutant virus that did not express NS2 did not replicate in HAE-ALI (Shen, Deng et al., 2015).
HAE-ALI cultures are commercially available. We have used the EpiAirway and MucilAir HAE purchased from MatTek Co. (Ashland, MA, USA) and also Epithelix from SàRL (Geneva, Switzerland), respectively. For those have we tested, they were susceptible to HBoV1 infection and demonstrated the hallmarks of airway epithelial damage (Deng, Li et al., 2014). However, the infectivity of HBoV1 in these HAE cultures was apparently poorer than in the primary HAE-ALI or the CuFi-ALI cultures that we generated in the lab (Deng, Li et al., 2014). At an MOI of 100, in the infected EpiAirway, the apical virus release reached the peak of 1× 106 gc/μl (200 μl of washes/insert) at 3 days post-infection, while in the MucilAir HAE, it reached a peak of 7 × 106 gc/μl at 9 days post-infection.
Critical Parameters and Troubleshooting
The donors of airway epithelial cells: We found that HAE-ALI cultures generated from airway epithelial cells of different donors often have varied infectivity of HBoV1. This is dependent on the degree of the differentiation of the cells. In general, airway cells isolated from young donors can be differentiated much better than these isolated from older donor (e.g. >60). We have obtained a stock of primary airway epithelial cells of a young donor of 4-years. These cells were expanded to the 20 passages in SAGM-H medium. They still differentiated well at an ALI and supported high HBoV1 infection.
Contamination of the ALI cultures: Since the cells are polarized for 3–4 weeks and the infected HAE will be maintained for a few weeks sometimes before harvesting, it is important not to get the ALI cultures contaminated. We usually use a combination of gentamicin (50 μg/ml) and amphotercin B (1.25 μg/ul) to prevent fungi contamination. However, if fungi contamination still appears, you can try the combination of fluconazole (50 ug/ul), gentamicin (50 μg/ml) and amphotericin B (1.25 μg/ml), which are especially effective for preventing fungi infection when culturing the epithelial cultures for extended periods of time.
Coating the flask and insert with collagen: It is critical to treat the flasks and inserts that are used for primary airway epithelial cells. Please wash off the leftover collagen after coating.
Understanding the Results
Virus production:
An amount of ~1 to 2 × 1012 vgc of purified HBoV1 in the fraction (1 ml) of CsCl that has a density of 1.40 g/ml will be produced from 5 150-mm plates of pIHBoV1-transfected HEK293. We do not recommend that you further band the virus on the CsCl gradient as this would inactivate the virus.
Preparations of ALI cultures:
After 3–4 weeks of differentiation, the ALI cultures will have a TEER of >1,000 to 2,000 Ω. Any cultures that have a TEER lower than 1,000 Ω are not recommended for use for virus infection. HAE-ALI cultures prepared from primary airway epithelial cells are dependent on the donor who donates the primary cells and on passages of the cells. CuFi-ALI mostly will have a lower TEER than those of primary HAE-ALI, whose TEER is dependent on the passage of the cells as well. We recommend not using the CuFi-8 cells over 30 passages.
Virus infection:
HBoV1 apically infects HAE-ALI cultures at an efficiency much higher than it does basolaterally. At MOIs ranging from 100 to 0.001 vgc/cell, apical infection of HBoV1 in primary HAE-ALI released virus at a peak titer of 107-108 vgc/μl (in 150 μl of apical wash of a 0.6 cm2 insert) ranging from days 3–24 post-infection (Deng, Yan et al., 2013). In a basolateral infection with an MOI of 1 vgc/cell, the virus release peaked at 107 vgc/μl and appeared at 16 days post-infection (Deng, Yan et al., 2013). HBoV1 infects CuFi-HAE poorer than primary HAE. At an apical MOI of 750 vgc/cell, HBoV1-infected CuFi-ALI released virus at the peak of 107 vgc/μl at 6 days post-infection, compared to the peak of 108 vgc/μl at 5 days post-infection from infected primary HAE-ALI (Huang, Deng et al., 2012). Commercially available HAE cultures can also be infected with HBoV1, but the infectivity was lower than in house made HAE cultures (Deng, Li et al., 2014).
The cells collected from the inserts that have the peak virus release should have over 60% of the cells infected as determined by staining of an anti-HBoV1 NS1 antibody by immunofluorescent assay. We found that the higher the apical virus release is, the higher the percentage of infected cells. We do not recommend collecting the infected cells after the peak of apical virus release, as severe cell death is induced by HBoV1 infection.
Time Considerations
It will take 2–3 months to complete this protocol for HBoV1 infection of polarized human airway epithelium. Basic Protocol 1 will take ~4 days to purify HBoV1 plasmid DNA and ~3 days to get the HEK293 cells transfected, and require one week to purify and quantify the progeny virions. Basic Protocol 2 typically take 4–6 weeks to get the fully differentiated HAE-ALI culture ready for virus infection. The expansion of CuFi-8 cells and human airway basal cells takes ~1–2 weeks and the polarization at an ALI takes 3–4 weeks. Basic Protocol 3 will take 1–2 weeks to have the polarized HAE-ALI infected and virus replication reached a maximum, and one week to analyze infected HAE-ALI. Overall, to complete this protocol, a time frame of 10–12 weeks should be scheduled.
ACKNOWLEDGMENTS
This study was supported by PHS grants AI150877 and AI139572 from the National Institute of Allergy and Infectious Diseases. This study was also supported by grant YAN19XX0 from the Cystic Fibrosis Foundation.
LITERATURE CITED
- Allander T, Jartti T, Gupta S, Niesters HG, Lehtinen P, Osterback R, Vuorinen T, Waris M, Bjerkner A, Tiveljung-Lindell A, van den Hoogen BG, Hyypiä T, Ruuskanen O, 2007. Human bocavirus and acute wheezing in children. Clin.Infect.Dis 44, 904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JC, Singh KK, Spector SA, Sawyer MH, 2006. Human bocavirus: prevalence and clinical spectrum at a children’s hospital. Clin.Infect.Dis 43, 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayora-Talavera G, Shelton H, Scull MA, Ren J, Jones IM, Pickles RJ, Barclay WS, 2009. Mutations in H5N1 influenza virus hemagglutinin that confer binding to human tracheal airway epithelium. PLoS.ONE 4, e7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banach S, Orenstein JM, Fox LM, Randell SH, Rowley AH, Baker SC, 2009. Human airway epithelial cell culture to identify new respiratory viruses: coronavirus NL63 as a model. J.Virol.Methods 156, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde I, Salmon P, Trono D, 2010. Production and titration of lentiviral vectors. Curr.Protoc.Neurosci Chapter 4:Unit 4.21. doi: 10.1002/0471142301.ns0421s53., Unit. [DOI] [PubMed] [Google Scholar]
- Blessing K, Neske F, Herre U, Kreth HW, Weissbrich B, 2009. Prolonged detection of human bocavirus DNA in nasopharyngeal aspirates of children with respiratory tract disease. Pediatr.Infect.Dis.J 28, 1018–1019. [DOI] [PubMed] [Google Scholar]
- Bruning AHL, Susi P, Toivola H, Christensen KK, Soderlund-Venermo M, Hedman K, Aarola H, Koskinen KK, Koskinen JO, 2016. Follow up of human bocavirus 1 infection in the nasopharynx by a new rapid antigen test. New Microbes and New Infections 11, 17–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo C, Garcia-Garcia ML, Pozo F, Carvajal O, Perez-Brena P, Casas I, 2008. Clinical characteristics of human bocavirus infections compared with other respiratory viruses in Spanish children. Pediatr.Infect.Dis.J 27, 677–680. [DOI] [PubMed] [Google Scholar]
- Choi EH, Lee HJ, Kim SJ, Eun BW, Kim NH, Lee JA, Lee JH, Song EK, Kim SH, Park JY, Sung JY, 2006. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000–2005. Clin.Infect.Dis 43, 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A, Døllner H, Shanke LH, Krokstad S, Moe N, Nordbø SA, 2013. Detection of spliced mRNA from human bocavirus 1 in clinical samples from children with respiratory tract infections. Emerg.Infect.Dis 19, 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A, Kesti O, Elenius V, Eskola AL, Dollner H, Altunbulakli C, Akdis CA, Soderlund-Venermo M, Jartti T, 2019. Human bocaviruses and paediatric infections. Lancet Child Adolesc.Health 3, 418–426. [DOI] [PubMed] [Google Scholar]
- Christensen A, Nordbø SA, Krokstad S, Rognlien AG, Døllner H, 2010. Human bocavirus in children: mono-detection, high viral load and viraemia are associated with respiratory tract infection. J.Clin.Virol 49, 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore SF, Agbandje-McKenna M, Canuti M, Chiorini JA, Eis-Hubinger AM, Hughes J, Mietzsch M, Modha S, Ogliastro M, Penzes JJ, Pintel DJ, Qiu J, Soderlund-Venermo M, Tattersall P, Tijssen P, Ictv Report Consortium, 2019. ICTV Virus Taxonomy Profile: Parvoviridae. J Gen.Virol 100, 367–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore SF, Tattersall P, 2005. A rolling-haipin strategy: basic mechanisms of DNA replication in the parvoviruses In: Kerr J, Cotmore SF, Bloom ME, Linden RM, Parrish CR (Eds.), Parvoviruses Hoddler Arond, London, pp. 171–181. [Google Scholar]
- Cotmore SF, Tattersall P, 2013. Parvovirus diversity and DNA damage responses. Cold Spring Harb.Perspect.Biol 5, a012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore SF, Tattersall P, 2014. Parvoviruses: Small Does Not Mean Simple. Annu.Rev.Virol 1, 517–537. [DOI] [PubMed] [Google Scholar]
- Del Rosal T, Garcia-Garcia ML, Calvo C, Gozalo F, Pozo F, Casas I, 2015. Recurrent wheezing and asthma after bocavirus bronchiolitis. Allergol.Immunopathol.(Madr.) 45, 410–414. [DOI] [PubMed] [Google Scholar]
- Deng X, Li Y, Qiu J, 2014. Human bocavirus 1 infects commercially available primary human airway epithelium cultures productively. J.Virol Methods 195, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Xu P, Zou W, Shen W, Peng J, Liu K, Engelhardt JF, Yan Z, Qiu J, 2016. DNA Damage Signaling Is Required for Replication of Human Bocavirus 1 DNA in Dividing HEK293 Cells. J Virol 91, e01831–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Yan Z, Cheng F, Engelhardt JF, Qiu J, 2016. Replication of an Autonomous Human Parvovirus in Non-dividing Human Airway Epithelium Is Facilitated through the DNA Damage and Repair Pathways. PLoS.Pathog 12, e1005399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Yan Z, Luo Y, Xu J, Cheng Y, Li Y, Engelhardt J, Qiu J, 2013. In vitro modeling of human bocavirus 1 infection of polarized primary human airway epithelia. J.Virol 87, 4097–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Zou W, Xiong M, Wang Z, Engelhardt JF, Ye SQ, Yan Z, Qiu J, 2017. Human Parvovirus Infection of Human Airway Epithelia Induces Pyroptotic Cell Death via Inhibiting Apoptosis. J.Virol 91, e01533–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkman R, Koekkoek SM, Molenkamp R, Schildgen O, van der Hoek L, 2009. Human bocavirus can be cultured in differentiated human airway epithelial cells. J.Virol 83, 7739–7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Amaral de LC, Amantea SL, Pilger DA, Cantarelli V, 2013. Clinical and epidemiologic profile of lower respiratory tract infections associated with human bocavirus. Pediatr.Pulmonol 48, 1112–1118. [DOI] [PubMed] [Google Scholar]
- Don M, Söderlund-Venermo M, Hedman K, Ruuskanen O, Allander T, Korppi M, 2011. Don’t forget serum in the diagnosis of human bocavirus infection. J.Infect.Dis 203, 1031–1032. [DOI] [PubMed] [Google Scholar]
- Don M, Söderlund-Venermo M, Valent F, Lahtinen A, Hedman L, Canciani M, Hedman K, Korppi M, 2010. Serologically verified human bocavirus pneumonia in children. Pediatr.Pulmonol 45, 120–126. [DOI] [PubMed] [Google Scholar]
- Donaldson EF, Yount B, Sims AC, Burkett S, Pickles RJ, Baric RS, 2008. Systematic assembly of a full-length infectious clone of human coronavirus NL63. J.Virol 82, 11948–11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edner N, Castillo-Rodas P, Falk L, Hedman K, Soderlund-Venermo M, Allander T, 2011. Life-threatening respiratory tract disease with human bocavirus-1 infection in a four-year-old child. J.Clin.Microbiol 50, 531–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essaidi-Laziosi M, Brito F, Benaoudia S, Royston L, Cagno V, Fernandes-Rocha M, Piuz I, Zdobnov E, Huang S, Constant S, Boldi MO, Kaiser L, Tapparel C, 2018. Propagation of respiratory viruses in human airway epithelia reveals persistent virus-specific signatures. J Allergy Clin.Immunol 141, 2074–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AM, Lu X, Chittaganpitch M, Peret T, Fischer J, Dowell SF, Anderson LJ, Erdman D, Olsen SJ, 2007. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J.Infect.Dis 195, 1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganaie SS, Qiu J, 2018. Recent Advances in Replication and Infection of Human Parvovirus B19. Front Cell Infect.Microbiol 8, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Deng X, Yan Z, Cheng F, Luo Y, Shen W, Lei-Butters DC, Chen AY, Li Y, Tang L, Söderlund-Venermo M, Engelhardt JF, Qiu J, 2012. Establishment of a reverse genetics system for studying human bocavirus in human airway epithelia. PLoS.Pathog 8, e1002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PB Jr., 2005. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol 79, 14614–14621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jula A, Waris M, Kantola K, Peltola V, Söderlund-Venerm M, Hedman K, Ruuskanen O, 2013. Primary and secondary human bocavirus 1 infections in a family, Finland. Emerg.Infect.Dis 19, 1328–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantola K, Hedman L, Allander T, Jartti T, Lehtinen P, Ruuskanen O, Hedman K, Söderlund-Venermo M, 2008. Serodiagnosis of human bocavirus infection. Clin.Infect.Dis 46, 540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalar L, Lindner J, Schimanski S, Kertai M, Segerer H, Modrow S, 2010. Prevalence and clinical aspects of human bocavirus infection in children. Clin.Microbiol.Infect 16, 633–639. [DOI] [PubMed] [Google Scholar]
- Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, Welsh MJ, 2002. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol.Biol 188, 115–137. [DOI] [PubMed] [Google Scholar]
- Keiser NW, Engelhardt JF, 2013. Gene delivery to the airway. Curr.Protoc.Hum.Genet. Chapter 13, Unit 13.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesebir D, Vazquez M, Weibel C, Shapiro ED, Ferguson D, Landry ML, Kahn JS, 2006. Human bocavirus infection in young children in the United States: molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J.Infect.Dis 194, 1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner RW, Soderlund-Venermo M, van Koningsbruggen-Rietschel S, Kaiser R, Malecki M, Schildgen O, 2011. Severe human bocavirus infection, Germany. Emerg.Infect.Dis 17, 2303–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner J, Karalar L, Zehentmeier S, Plentz A, Pfister H, Struff W, Kertai M, Segerer H, Modrow S, 2008. Humoral Immune Response Against Human Bocavirus VP2 Virus-Like Particles. Viral Immunol 21, 443–449. [DOI] [PubMed] [Google Scholar]
- Longtin J, Bastien M, Gilca R, Leblanc E, de Serres G, Bergeron MG, Boivin G, 2008. Human bocavirus infections in hospitalized children and adults. Emerg.Infect.Dis 14, 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Chittaganpitch M, Olsen SJ, Mackay IM, Sloots TP, Fry AM, Erdman DD, 2006. Real-time PCR assays for detection of bocavirus in human specimens. J Clin.Microbiol 44, 3231–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning A, Russell V, Eastick K, Leadbetter GH, Hallam N, Templeton K, Simmonds P, 2006. Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. J.Infect.Dis 194, 1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell H, Levin D, Forrest S, Beauchemin CA, Tipper J, Knight J, Donart N, Layton RC, Pyles J, Gao P, Harrod KS, Perelson AS, Koster F, 2011. Higher level of replication efficiency of 2009 (H1N1) pandemic influenza virus than those of seasonal and avian strains: kinetics from epithelial cell culture and computational modeling. J.Virol 85, 1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama Y, Hamada H, Okada M, Tsuchiya N, Maru H, Shirato Y, Maeda Y, Hirose Y, Yoshida M, Omura Y, Honda T, Muto A, Hayashi K, Terai M, 2010. Distinctive clinical features of human bocavirus in children younger than 2 years. Eur.J Pediatr 169, 1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H, Vinarsky V, Tata PR, Brazauskas K, Choi SH, Crooke AK, Zhang B, Solomon GM, Turner B, Bihler H, Harrington J, Lapey A, Channick C, Keyes C, Freund A, Artandi S, Mense M, Rowe S, Engelhardt JF, Hsu YC, Rajagopal J, 2016. Dual SMAD Signaling Inhibition Enables Long-Term Expansion of Diverse Epithelial Basal Cells. Cell Stem Cell 19, 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento-Carvalho CM, Cardoso MR, Meriluoto M, Kemppainen K, Kantola K, Ruuskanen O, Hedman K, Söderlund-Venermo M, 2012. Human bocavirus infection diagnosed serologically among children admitted to hospital with community-acquired pneumonia in a tropical region. J.Med.Virol 84, 253–258. [DOI] [PubMed] [Google Scholar]
- Neske F, Blessing K, Tollmann F, Schubert J, Rethwilm A, Kreth HW, Weissbrich B, 2007. Real-time PCR for diagnosis of human bocavirus infections and phylogenetic analysis. J Clin.Microbiol 45, 2116–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo LM, Porotto M, Yokoyama CC, Palmer SG, Mungall BA, Greengard O, Niewiesk S, Moscona A, 2009. Human parainfluenza virus infection of the airway epithelium: viral hemagglutinin-neuraminidase regulates fusion protein activation and modulates infectivity. J.Virol 83, 6900–6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo F, Garcia-Garcia ML, Calvo C, Cuesta I, Perez-Brena P, Casas I, 2007. High incidence of human bocavirus infection in children in Spain. J.Clin.Virol 40, 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proenca-Modena JL, Gagliardi TB, Escremim de PF, Iwamoto MA, Criado MF, Camara AA, Acrani GO, Cintra OA, Cervi MC, de Paula Arruda LK, Arruda E, 2011. Detection of human bocavirus mRNA in respiratory secretions correlates with high viral load and concurrent diarrhea. PLoS.ONE 6, e21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrc K, Sims AC, Dijkman R, Jebbink M, Long C, Deming D, Donaldson E, Vabret A, Baric R, van der Hoek L, Pickles R, 2010. Culturing the unculturable: human coronavirus HKU1 infects, replicates, and produces progeny virions in human ciliated airway epithelial cell cultures. J.Virol 84, 11255–11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Söderlund-Venermo M, Young NS, 2017. Human parvoviruses. Clin.Microbiol.Rev 30, 43–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezes S, Soderlund-Venermo M, Roivainen M, Kemppainen K, Szabo Z, Sziklai I, Pitkaranta A, 2009. Human bocavirus and rhino-enteroviruses in childhood otitis media with effusion. J Clin.Virol 46, 234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruohola A, Waris M, Allander T, Ziegler T, Heikkinen T, Ruuskanen O, 2009. Viral etiology of common cold in children, Finland. Emerg.Infect.Dis 15, 344–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildgen O, 2010. Human bocavirus: increasing evidence for virulence. Pediatr.Pulmonol 45, 118–119. [DOI] [PubMed] [Google Scholar]
- Schildgen O, Muller A, Allander T, Mackay IM, Volz S, Kupfer B, Simon A, 2008. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin.Microbiol.Rev 21, 291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaberg R, Queen K, Simmon K, Tardif K, Stockmann C, Flygare S, Kennedy B, Voelkerding K, Bramley A, Zhang J, Eilbeck K, Yandell M, Jain S, Pavia AT, Tong S, Ampofo K, 2017. Viral Pathogen Detection by Metagenomics and Pan Viral Group PCR in Children with Pneumonia Lacking Identifiable Etiology. J.Infect.Dis 215, 1407–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scull MA, Gillim-Ross L, Santos C, Roberts KL, Bordonali E, Subbarao K, Barclay WS, Pickles RJ, 2009. Avian Influenza virus glycoproteins restrict virus replication and spread through human airway epithelium at temperatures of the proximal airways. PLoS.Pathog 5, e1000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan TP, Sims AC, Zhou S, Graham RL, Pruijssers AJ, Agostini ML, Leist SR, Schafer A, Dinnon KH III, Stevens LJ, Chappell JD, Lu X, Hughes TM, George AS, Hill CS, Montgomery SA, Brown AJ, Bluemling GR, Natchus MG, Saindane M, Kolykhalov AA, Painter G, Harcourt J, Tamin A, Thornburg NJ, Swanstrom R, Denison MR, Baric RS, 2020. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci.Transl.Med Online ahead of print, -PMCID: PMC7164393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Deng X, Zou W, Cheng F, Engelhardt JF, Yan Z, Qiu J, 2015. Identification and Functional Analysis of Novel Non-structural Proteins of Human Bocavirus 1. J.Virol 89, 10097–10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Deng X, Zou W, Engelhardt JF, Yan Z, Qiu J, 2016. Analysis of the Cis and Trans Requirements for DNA Replication at the Right End Hairpin of the Human Bocavirus 1 Genome. J Virol 90, 7761–7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims AC, Baric RS, Yount B, Burkett SE, Collins PL, Pickles RJ, 2005. Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: role of ciliated cells in viral spread in the conducting airways of the lungs. J.Virol 79, 15511–15524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund-Venermo M, Lahtinen A, Jartti T, Hedman L, Kemppainen K, Lehtinen P, Allander T, Ruuskanen O, Hedman K, 2009. Clinical assessment and improved diagnosis of bocavirus-induced wheezing in children, Finland. Emerg.Infect.Dis 15, 1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabai J, Fakhiri J, Meyburg J, Linse KP, Xu M, Soderlund-Venermo M, Grimm D, Schnitzler P, 2019. Severe Human Bocavirus 1 Respiratory Tract Infection in an Immunodeficient Child With Fatal Outcome. Pediatr.Infect.Dis.J 10. [DOI] [PubMed] [Google Scholar]
- Terrosi C, Fabbiani M, Cellesi C, Cusi MG, 2007. Human bocavirus detection in an atopic child affected by pneumonia associated with wheezing. J.Clin.Virol 40, 43–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursic T, Jevsnik M, Zigon N, Krivec U, Beden AB, Praprotnik M, Petrovec M, 2012. Human bocavirus and other respiratory viral infections in a 2-year cohort of hospitalized children. J.Med.Virol 84, 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursic T, Krivec U, Kalan G, Petrovec M, 2015. Fatal human bocavirus infection in an 18-month-old child with chronic lung disease of prematurity. Pediatr.Infect.Dis.J 34, 111–112. [DOI] [PubMed] [Google Scholar]
- Ursic T, Steyer A, Kopriva S, Kalan G, Krivec U, Petrovec M, 2011. Human bocavirus as the cause of a life-threatening infection. J.Clin.Microbiol 49, 1179–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villenave R, Thavagnanam S, Sarlang S, Parker J, Douglas I, Skibinski G, Heaney LG, McKaigue JP, Coyle PV, Shields MD, Power UF, 2012. In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo. Proc.Natl.Acad.Sci.U.S.A 109, 5040–5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Deering C, Macke M, Shao J, Burns R, Blau DM, Holmes KV, Davidson BL, Perlman S, McCray PB Jr., 2000. Human coronavirus 229E infects polarized airway epithelia from the apical surface. J.Virol 74, 9234–9239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Wang W, Yan H, Ren P, Zhang J, Shen J, Deubel V, 2010. Correlation between bocavirus infection and humoral response, and co-infection with other respiratory viruses in children with acute respiratory infection. J.Clin.Virol 47, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Deng X, Zou W, Engelhardt JF, Yan Z, Qiu J, 2017. Human Bocavirus 1 Is a Novel Helper for Adeno-Associated Virus Replication. J.Virol 91, e00710–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Shen W, Cheng F, Deng X, Engelhardt JF, Yan Z, Qiu J, 2017. Parvovirus Expresses a Small Noncoding RNA That Plays an Essential Role in Virus Replication. J.Virol 91, e02375–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Arku B, Jartti T, Koskinen J, Peltola V, Hedman K, Soderlund-Venermo M, 2017. Comparative diagnosis of human bocavirus 1 respiratory infection by mRNA RT-PCR, DNA quantitative PCR and serology. J.Infect.Dis 215, 1551–1557. [DOI] [PubMed] [Google Scholar]
- Zabner J, Karp P, Seiler M, Phillips SL, Mitchell CJ, Saavedra M, Welsh M, Klingelhutz AJ, 2003. Development of cystic fibrosis and noncystic fibrosis airway cell lines. Am.J.Physiol Lung Cell Mol.Physiol 284, L844–L854. [DOI] [PubMed] [Google Scholar]
- Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ, 2002. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J.Virol 76, 5654–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Yu X, Wang C, Teng Z, Wang C, Shen J, Gao Y, Zhu Z, Wang J, Yuan Z, Wu F, Zhang X, Ghildyal R, 2013. High human bocavirus viral load is associated with disease severity in children under five years of age. PLoS.ONE 8, e62318. [DOI] [PMC free article] [PubMed] [Google Scholar]


