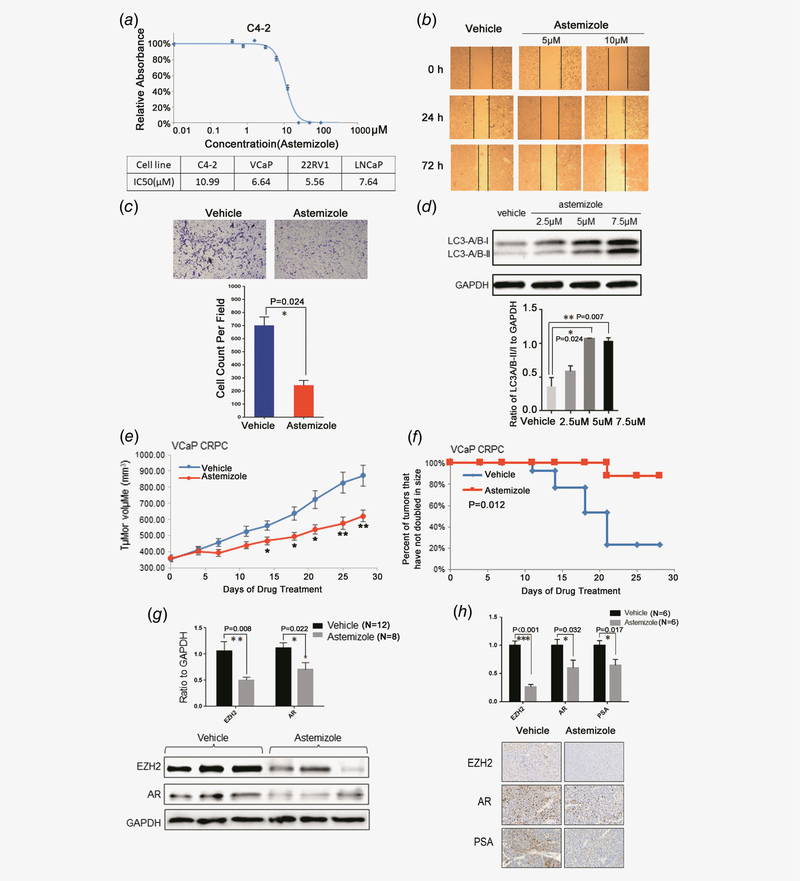
Figure 5.
Astemizole has potent therapeutic effects on prostate cancer. (a) Astemizole critically thwarts cell proliferation in C4–2 and other AR-positive prostate cancer cell lines. (b) The wound healing assay indicates that astemizole compromises the migration of C4–2 cells. (c) Astemizole decreases the invasive abilities of C4–2 cells compared to vehicle treatment. Cell count was analyzed and the difference was statistically significant. (d)C4–2 cells were treated with 2.5, 5 and 7.5 μM of astemizole. Cells were lysed 48 hr after treatment and blotted with anti-LC3-A/B antibody. The ratio of LC3-A/B-II/I to GAPDH was elevated as dose increased, which indicates that astemizole induces autophagy in prostate cancer cells. (e) Castration-resistant VCaP xenograft mouse models were generated. Castrated mice bearing CPRC xenografts received vehicle or astemizole treatment (50 mg kg−1) daily (5 days per week). Caliper measurements were taken every 4 days to determine tumor volume. Mean tumor volume SEM, *p < 0.05, **p < 0.01 vs. vehicle was marked. (f) Kaplan–Meier survival plot compares progression-free survival. (g) Upper panel: Proteins were blotted and quantitated to compare the protein levels of EZH2 and AR in astemizole-treated group (n = 8) compared to vehicle-treated group (n = 12). Lower panel: The expression of EZH2 and AR was decreased in response to astemizole treatment. (h) The proportion of the cells stained with EZH2/AR/PSA in astemizole-treated group (n = 6) were significantly lower than that in vehicle-treated group (n = 6).