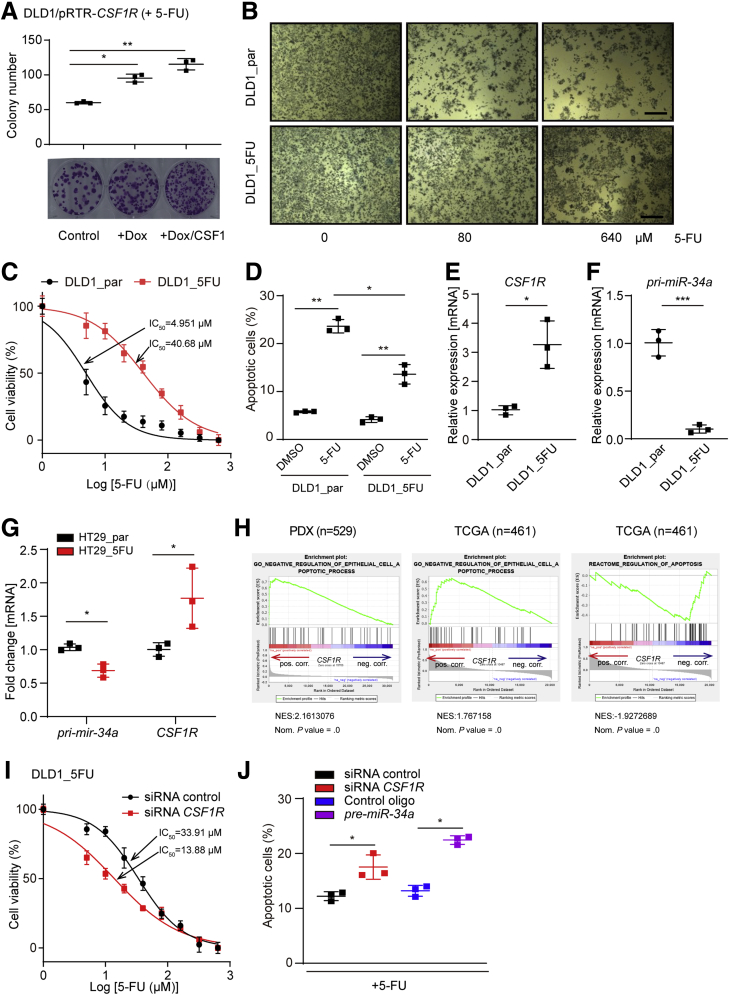
Figure 10.
Interdependent deregulation of miR-34a and CSF1R mediates resistance to 5-FU. (A) For a colony-formation assay 500 cells were seeded per well of a 6-well plate and cultivated with or without Dox for 24 hours, then exposed to CSF1 for another 24 hours, and then treated with 5-FU for 72 hours. Subsequently, cells were fixed and stained with crystal violet. Quantification of colony formation (upper panel) and representative examples of crystal violet staining (lower panel). (B) The indicated cell pools were treated with 5-FU for 48 hours and subsequently subjected to an MTT assay. Micrographs show cell pools with formation of MTT formazan, which is directly proportional to the number of living cells Scale bars = 200 μm. (C) IC50 determination of DLD1_par and DLD1_5FU cells in response to 5-FU. Cells were treated with the indicated concentrations of 5-FU for 48 hours and then subjected to an MTT assay. (D) Detection of apoptotic cells by Annexin V-FITC and PI staining after treatment with 5-FU for 36 hours. qPCR analysis of (E) CSF1R and (F) pri-miR34a expression in DLD1_par and DLD1_5FU cells. (G) Detection of pri-miR-34a and CSF1R expression in HT29_par and HT29_5FU cells. (H) Genes were preranked by expression correlation coefficient (Pearson r) with CSF1R in descending order from left (positive correlation) to right (negative correlation) based on RNA expression data, and association of the indicated gene signatures with CSF1R expression was analyzed by GSEA. (I) DLD1_5FU cells were transfected with control or CSF1R-specific siRNAs for 24 hours and subsequently treated with increasing concentrations of 5-FU for 48 hours. Then the IC50 was determined by an MTT assay. (J) DLD1_5FU cells were transfected with indicated oligonucleotides, and subsequently treated with 5-FU for 36 hours, and apoptotic cells were detected by Annexin V-FITC and PI staining. In panels A, D, E, F, G, and J, mean values ± SD are provided. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.