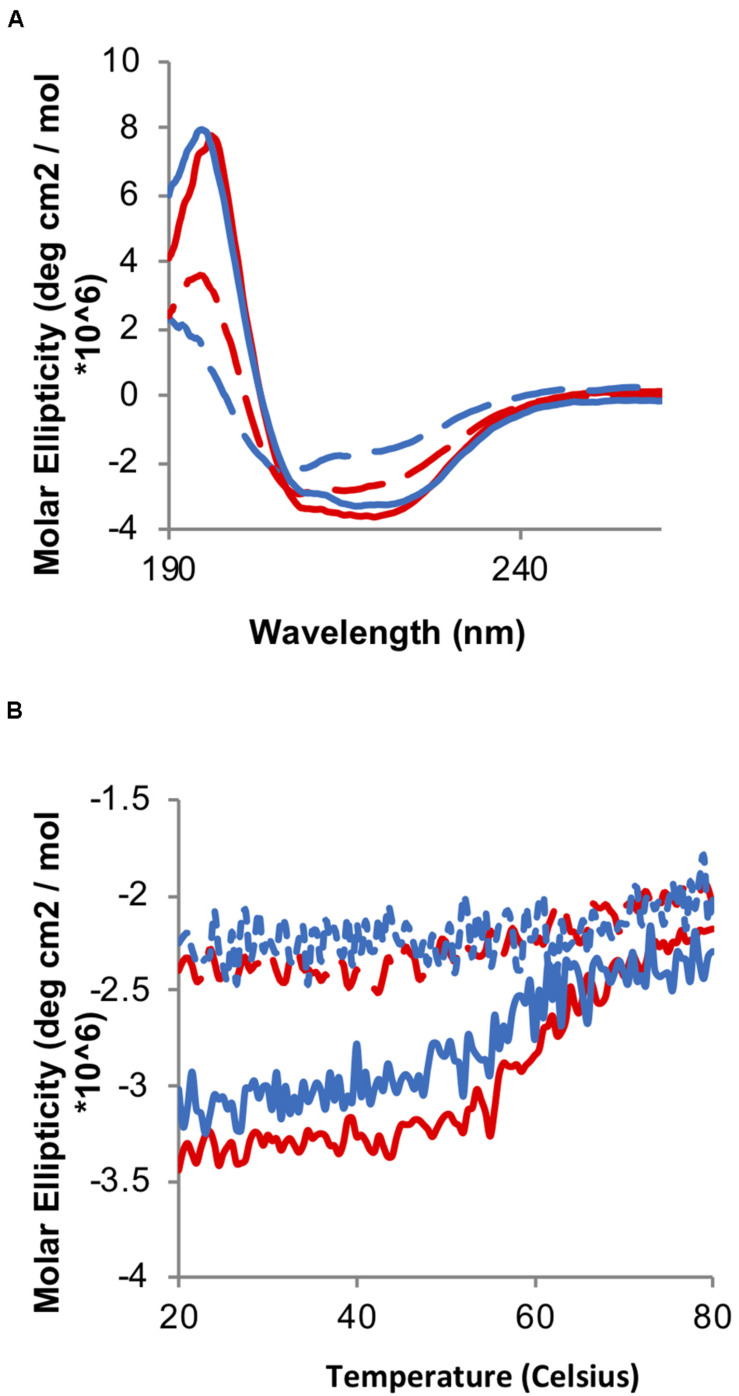
FIGURE 5.
Oxidation-induced unfolding of the wild type and Hsp33-TrypOx variant. (A) Far-UV CD spectra of inactive reduced (solid line) and active oxidized (dashed line) wild type Hsp33 (red) and Hsp33-TrypOx proteins (blue) (5 μM). (B) Thermal stability of wild type Hsp33 (red) and Hsp33-TrypOx (blue) in either reduced (solid) or oxidized (dashed) forms was determined by monitoring the changes in molecular ellipticity at 217 nm. The proteins were heated from 20 to 80°C at 1°C/min.