Abstract
Observing different kinetics of nutrient-induced insulin secretion in fresh and cultured islets under the same condition we compared parameters of stimulus secretion coupling in freshly isolated and 22-h-cultured NMRI mouse islets. Stimulation of fresh islets with 30 mM glucose after perifusion without nutrient gave a continuously ascending secretion rate. In 22-h-cultured islets the same protocol produced a brisk first phase followed by a moderately elevated plateau, a pattern regarded to be typical for mouse islets. This was also the response of cultured islets to the nutrient secretagogue alpha-ketoisocaproic acid, whereas the secretion of fresh islets increased similarly fast but remained strongly elevated. The responses of fresh and cultured islets to purely depolarizing stimuli (tolbutamide or KCl), however, were closely similar. Signs of apoptosis and necrosis were rare in both preparations. In cultured islets, the glucose-induced rise of the cytosolic Ca2+ concentration started from a lower value and was larger as was the increase of the ATP/ADP ratio. The prestimulatory level of mitochondrial reducing equivalents, expressed as the NAD(P)H/FAD fluorescence ratio, was lower in cultured islets, but increased more strongly than in fresh islets. When culture conditions were modified by replacing RPMI with Krebs–Ringer medium and FCS with BSA, the amount of released insulin varied widely, but the kinetics always showed a predominant first phase. In conclusion, the secretion kinetics of fresh mouse islets is more responsive to variations of nutrient stimulation than cultured islets. The more uniform kinetics of the latter may be caused by a different use of endogenous metabolites.
Keywords: cytosolic calcium concentration, glucose, insulin secretion, metabolic amplification, mitochondria
Introduction
The endocrine pancreas responds to an increase in ambient glucose with a biphasic pattern of insulin release, represented by a short (5–10 min) first phase, followed by a long, sustained second phase release. This unique feature is best shown in response to a ‘square wave’ glucose stimulus, either in vivo or in vitro (1, 2). It is obvious that such a stimulation pattern is non-physiological, but the resulting biphasic response is the hallmark of the healthy, metabolically well-coupled endocrine pancreas. In type 2 diabetes or in models of this disease, it displays varying degrees of a diminished insulin response, usually described to be more prominent during the first phase, but also recognizable during the second phase (3, 4, 5).
The mechanisms underlying the glucose-induced biphasic pattern of insulin secretion are still incompletely understood in spite of intensive research activities in the area. Heterogeneity within the insulin granule pool and/or heterogeneity of the signals in stimulus-secretion coupling may both contribute (6, 7). Nutrient secretagogues (glucose and a limited number of amino- and keto acids) act via a bifurcating pathway. The oxidative phosphorylation induces the electrical activity by closure of the ATP-sensitive K+ channels (KATP channels), additional signals from the mitochondrial metabolism act further downstream (8).
Since depolarization-induced Ca2+ influx is considered to represent the ‘triggering’ signal of nutrient-stimulated insulin secretion it is often assumed that the first phase is exclusively determined by the opening of voltage-dependent Ca2+ channels leading to the fusion of membrane-docked granules (9). The second phase in contrast would then be formed by the additional signals, which are referred to as ‘amplifying pathway’ (8). However, the characteristics of both first phase and second phase secretion were markedly influenced by modulating the pre-stimulatory conditions so as to affect the amplifying pathway (10, 11).
The effect of the amplifying pathway on the level of insulin secretion can be demonstrated by increasing the concentration of a nutrient secretagogue when the beta cells are already depolarized, either by pharmacological KATP channel closure (12) or by K+ depolarization plus pharmacological KATP channel opening (13). When the KATP channel closure takes place in the absence of exogenous nutrient, the metabolic amplification by a subsequent glucose stimulation is lost; however, the metabolic amplification by the keto acid alpha-ketoisocaproic acid (KIC) remains intact (14). This discrepancy between the amplifying effect of two nutrient secretagogues may lead to the unambiguous identification of the relevant signaling metabolites in this pathway, which has not yet been achieved (15, 16).
Investigating the mechanisms leading to the glucose-selective loss of the metabolic amplification, we observed that a marked difference existed between the response of freshly isolated islets and that of cultured islets. So we tested the hypothesis that the cell culture phase, which is often routinely employed after collagenase digestion of the islets (17) does not just re-establish pre-isolation conditions, but leaves its mark on the metabolic signaling in the beta cells and, in consequence, the kinetics of nutrient-induced insulin secretion.
Materials and methods
Chemicals
Collagenase for islet isolation was initially NB8 from Serva (Heidelberg, Germany), in later experiments collagenase P from Roche (Sigma) was used. Fura-2 LeakRes (AM) was obtained from TEF-Labs (Austin, TX, USA). Cell culture medium RPMI 1640 without glucose was from Sigma and fetal calf serum (FCS Gold ADD) was from Bio & Sell (Nürnberg-Feucht, Germany). BSA (fraction V) and all other reagents of analytical grade were from E. Merck.
Tissue and tissue culture
Islets were isolated from the pancreas of female NMRI mice (12–16 weeks old, fed ad libitum) by a collagenase digestion technique and hand-picked under a stereomicroscope (for details see (18)). For some of the experiments the islets were microdissected to avoid contact with the collagenase. Care was taken to limit the time between onset of digestion and start of the perifusion of freshly isolated islets to 45 min. For a direct comparison between freshly isolated islets and cultured islets the same batch of islets was split in two. Islets were cultured in cell culture medium RPMI 1640 and 10% fetal calf serum (vol/vol) in a humidified atmosphere of 95% air and 5% CO2 at 37°C. In some of the experiments FCS was replaced by 0.4% (w/v) BSA since this concentration comes close to the protein concentration of a medium with 10% FCS supplementation. The glucose concentration during culture was set at 5 mM (by adding glucose to glucose-free RPMI), except when specifically stated otherwise. The culture duration was always 22 h. Animal care was supervised by the regional authority (LAVES, Lower Saxony, Germany) and conformed to the current EU regulations.
Integrity of freshly isolated and cultured islets
The appearance of freshly isolated islets and cultured islets was checked using transmitted light microscopy with DIC-contrast. Islets were placed in an open chamber with glass bottom and a water immersion objective (Zeiss Achroplan 40×, 0.75 w) was used for inspection. Photomicrographs were taken by a Panasonic Lumix GX80 at the photo port of the upright Zeiss Axioscope FS microscope. The relation of live and dead cells in the islets was assessed by the live/dead assay according to the manufacturer’s protocol (PromoCell, Heidelberg, Germany). The non-fluorescent membrane-permeable Calcein AM-ester is cleaved in the cytosol of living cells whereby a green fluorescence is produced and retained within the cell when the plasma membrane is intact. Ethidium homodimer III is excluded from a living cell with an intact plasma membrane but can reach the nucleus of dead cells. There it binds to nuclear DNA whereby the red fluorescence is about 40-fold intensified. The occurrence of early steps of apoptosis in fresh and cultured islets was checked by annexin V assay (ABP Biosciences, Rockwell, MD, USA). Andy Fluor 488-coupled annexin V binds to the outer leaflet of the plasma membrane if phosphatidylserine is present, which indicates the onset of apoptosis (19). The number of dead cells is again indicated by the red fluorescence of ethidium homodimer III. After loading with the indicators, islets were placed on Petri dishes with glass bottom (ibidi GmbH, Gräfelfing, Germany) and placed on the stage of an inverted Nikon Ti2-E microscope fitted with a Yokogawa CSU W1 spinning disk unit. The green fluorescence of calcein and of Alexa Fluor 488 was excited at 491 nm, the red fluorescence of ethidium homodimer at 561 nm and collected by a Nikon CFI Plan Apochromat Lambda S40 XC Sil objective (40×, 1.25 N.A.), which is designed to image thick specimen of living cells. Images were acquired by a sCMOS camera (Prime BSI, Teledyne Photometrics, Tucson, AZ, USA) under control of Visiview Premier software (Visitron Systems, Munich, Germany).
Measurement of insulin secretion and insulin content
Batches of 50 islets were introduced into a purpose-made perifusion chamber (37°C) and perifused at 0.9 mL/min with a HEPES-buffered KR medium (NaCl 118.5 mM, KCl 4.7 mM, CaCl2 2.5 mM, KH2PO4 1.2 mM, MgSO4 1.2 mM, NaHCO3 20 mM, HEPES 10 mM, BSA 0.2%, w/v) which was saturated with 95% O2 and 5% CO2 and contained the respective secretagogue. This medium was also used for the microfluorometric measurements. The insulin content in the fractionated efflux was determined by ELISA according to the manufacturer’s protocol (Mercodia, Uppsala, Sweden). The islet insulin content was measured by sonicating a group of 30 islets in an ice-cooled microtube for 20 s and measuring the insulin concentration after appropriate dilution with the zero buffer of the ELISA set.
Measurement of islet NAD(P)H- and FAD-autofluorescence
The islet autofluorescence of NAD(P)H (NADH + NADPH) and of FAD (20, 21) was simultaneously recorded. One islet was perifused in a purpose-made chamber on the stage of an Orthoplan epifluorescence microscope (Leitz/Leica) with KR-medium at 0.2 mL/min and 35°C. After selecting a subregion of the islet fluorescence was excited with a 150W xenon arc using the following filter combinations (Omega Optical, Brattleboro, VT, USA): for NAD(P)H, excitation 366 ± 15 nm bandpass, dichroic separation 405 nm, emission 450 ± 32 nm bandpass; for FAD, excitation 440 ± 21 nm bandpass, dichroic separation 455 nm, emission 520 ± 20 nm bandpass. The filter cubes were switched every 2.5 s with an exposure time of 0.1 s. The fluorescence was collected by a Zeiss Fluar (10×, 0.5 N.A.) and quantified by a photon-counting multiplier. To calculate mean values, the fluorescence intensities were normalized to 100% at the last prestimulatory time point in each single experiment. Since
Simultaneous measurement of the cytosolic Ca2+ concentration ([Ca2+]i) and insulin secretion
Freshly isolated or cultured islets were incubated in KR medium (5 mM glucose) with Fura-2 LeakRes (AM) at a concentration of 2 µM for 45 min at 37°C. Five islets were then inserted in a temperature-controlled perifusion chamber (35°C) on the stage of a Zeiss Axiovert 135 microscope equipped with a Zeiss Fluar (10×, 0.5 N.A.) objective and perifused with KR-medium. The fluorescence of each islet (excitation at 340 or 380 nm, dichroic separation at 400 nm, emission 510 ± 40 nm bandpass) was recorded with a cooled CCD camera (Pursuit, Diagnostics Instruments, Sterling Heights, MI, USA) and evaluated using Visiview software (Visitron, Munich, Germany). The insulin content of the fractionated efflux was determined by ELISA (Mercodia, Uppsala, Sweden).
Islet content of adenine nucleotides
Fifteen cultured or fresh islets were statically incubated to mimic typical perifusion conditions. Thereafter, proteins were precipitated and the adenine nucleotides were extracted as described (14). ATP was determined by use of the luciferase method. The ADP content of the extract was converted into ATP by the pyruvate kinase reaction, the difference between both measurements yielding the net ADP content. Because of the interindividual variations in the adenine nucleotide contents the incubations were strictly performed in parallel and comparison between cultured and fresh islets was only made with islets from the same isolation batch.
Statistics
Results are presented as mean ± s.e.m. GraphPad Prism5 software (GraphPad) was used for statistic calculations and non-linear curve fitting (ELISA). If not specified otherwise, ‘significant’ refers to P < 0.05, unpaired, two-sided t-test.
Results
Cultured and fresh islets respond differently to the same stimulation
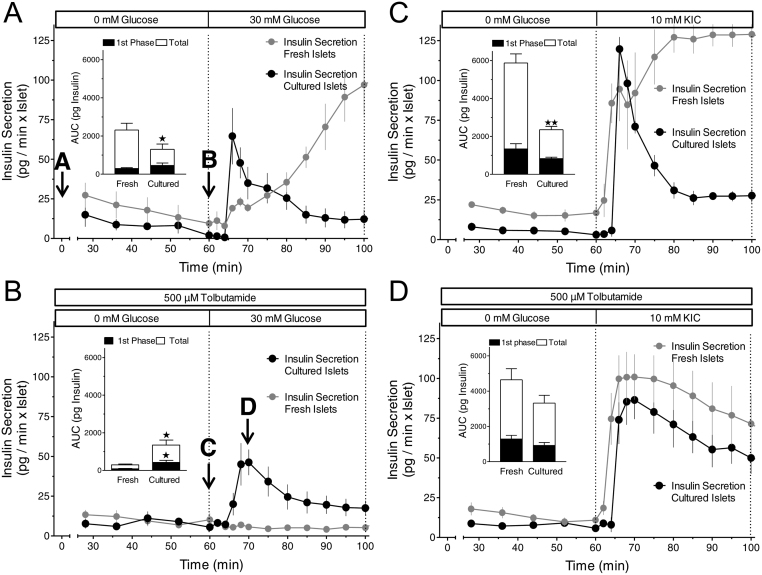
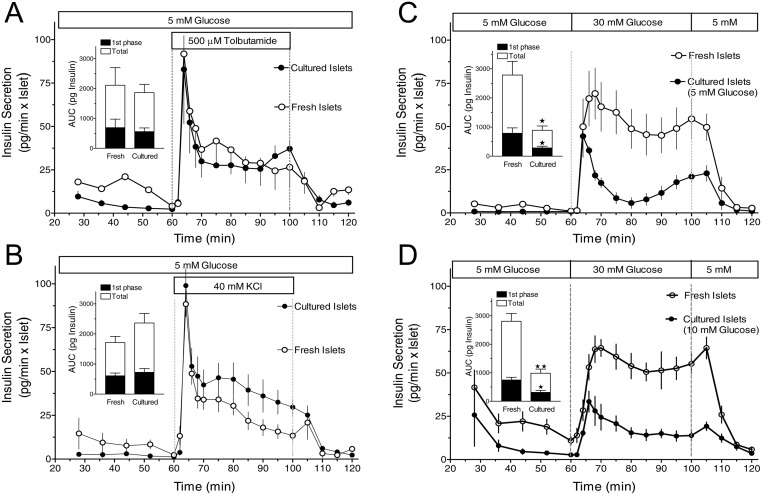
As shown previously (14) 30 mM glucose induced a modest first and pronounced second phase insulin secretion from freshly isolated islets after 60 min absence of exogenous nutrients (Fig. 1A) and was even unable to stimulate secretion when the islets were depolarized by tolbutamide during the initial nutrient deficiency (Fig. 1B). These initial experimental conditions had much less effect on KIC-stimulated insulin secretion, which was pronounced irrespective of whether or not the initial period of nutrient deficiency was combined with tolbutamide (Fig. 1C and D). We now unexpectedly observed that the loss of glucose-stimulated secretion did not occur when the initial nutrient deficiency in the presence of tolbutamide was preceded by 22-h islet culture (Fig. 1B). Islet culture also changed the kinetics of glucose- and KIC-induced secretion after nutrient deficiency. In both cases (Fig. 1A and C) the secretory response switched from dominating second phase to dominating first phase secretion. Only in the case of KIC-stimulation after preceding depolarization in the absence of exogenous nutrient the secretion pattern was qualitatively similar to that of freshly isolated islets (Fig. 1D).
Figure 1.
Predominance of the first phase response in cultured mouse islets (black symbols) as compared to the variable response pattern in freshly isolated mouse islets (grey symbols). (A) 22-h-cultured islets were perifused with Krebs–Ringer medium containing 0 mM glucose for 60 min, then 30 mM glucose was present for 40 min. The increase of the glucose concentration from 0 to 30 mM glucose elicited virtually opposite kinetics of secretion in fresh and in cultured islets. (B) Same protocol as in A with 500 µM tolbutamide additionally present throughout the perifusion. Note a virtually complete loss of glucose stimulation with fresh islets but not with cultured islets. (C) Same protocol as in A except for using 10 mM KIC instead of 30 mM glucose as the nutrient secretagogue. The KIC-induced high level of secretion was only maintained in fresh islets. (D) Same protocol as in B except for using 10 mM KIC instead of 30 mM glucose as the nutrient secretagogue. Note the robust response of fresh islets to KIC stimulation as compared to the loss of glucose stimulation in B. Values are means ± s.e.m. of 4–5 experiments each. The inset shows the integrated insulin secretion from 60 to 75 min (first phase) and from 60 to 100 min (total). *P < 0.05, **P < 0.01, cultured vs fresh islets under the same condition, unpaired two-sided t-test. The secretion data obtained with fresh islets are taken from Schulze et al. (16).
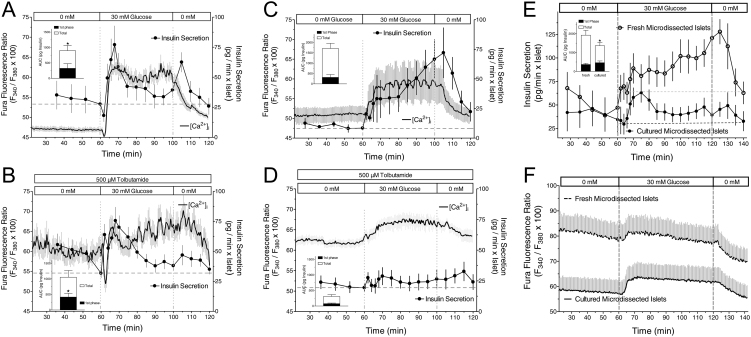
Similar kinetics of [Ca2+]i but not of insulin secretion during glucose stimulation in cultured and fresh islets
To assess the role of the cytosolic Ca2+ concentration ([Ca2+]i) and to verify the different responses of fresh and cultured islets, [Ca2+]i in perifused islets was measured simultaneously with the insulin secretion (Fig. 2). Elevating glucose from 0 to 30 mM produced a peak of secretion of cultured islets during the first 10 min, concurrent with a steep increase of [Ca2+]i, which was preceded by a modest transient decrease of both [Ca2+]i and secretion (Fig. 2A). The tolbutamide-induced depolarization in the absence of nutrient was clearly effective to sustain elevated [Ca2+]i levels. Under this condition 30 mM glucose initially caused a deep decrease of [Ca2+]i before eliciting a steep increase. The peak of secretion had the same height and occurred at the same time as under control condition (Fig. 2B).
Figure 2.
Simultaneous measurement of the cytosolic Ca2+ concentration and insulin secretion by cultured (A and B) or freshly isolated islets (C and D) in response to glucose. Fura-2-loaded islets were perifused with Krebs–Ringer medium containing 0 mM glucose (A and C) or 0 mM glucose plus 500 µM tolbutamide (B and D) for 60 min, then glucose was raised to 30 mM for 40 min and washed out thereafter. The Fura fluorescence ratio of three to five islets was measured per experiment, the secretion was measured in the fractionated efflux of these islets. Upon glucose stimulation the cultured islets show a plateau-like second phase after a predominant first phase. Freshly isolated islets show an ascending second phase (C) or a virtual loss of the insulinotropic effect of glucose (D). The difference in the secretion kinetics of the second phase is not paralleled by different kinetics of the Fura ratio value (compare A and C). Values are means ± s.e.m. of 4–7 experiments. The experiments shown in A and C were repeated with microdissected islets (E and F). Note again the different kinetics of the second phase secretion (E) in cultured (closed circles) as compared with freshly isolated islets (open circles), which is not reflected by the respective Fura ratio values (F). Values are means ± s.e.m. of seven experiments each. The inset shows the integrated insulin secretion from 60 to 75 min (first phase) and from 60 min to washout (total). *P < 0.05, cultured vs fresh islets under the same condition, unpaired two-sided t-test.
With freshly isolated islets 30 mM glucose led to a response with a moderate first phase and a continuously ascending second phase (Fig. 2C). The prestimulatory [Ca2+]i level was higher than in cultured islets, and the increase by glucose was less steep but reached a similar extent (compare Fig. 2A and C). In fresh islets the tolbutamide-induced depolarization in the absence of nutrient led to a strong inhibition of the secretory response to subsequent glucose stimulation (Fig. 2D). Tolbutamide led to prestimulatory [Ca2+]i levels which were similar as those in cultured islets (compare Fig. 2B and D). While the [Ca2+]i increase in response to 30 mM glucose was slow the levels attained were not less than under control condition.
To check for a specific role of collagenase-induced defects in shaping the secretion pattern of freshly isolated islets the effect of glucose on microdissected and cultured microdissected islets was compared (Fig. 2E and F). In response to 30 mM glucose fresh microdissected islets showed a continuously ascending second phase, whereas cultured islets from the same batch showed a predominant first phase followed by a moderately elevated plateau (Fig. 2E). Again the [Ca2+]i values in fresh islets were higher than in cultured islets, but both were higher than those of the corresponding collagenase-isolated islets (Fig. 2F, compare with Fig. 2A and C). This was likely caused by damaged residual exocrine cells still attached to the islets (Supplementary Fig. 1, see section on supplementary materials given at the end of this article). Upon glucose stimulation, the [Ca2+]i pattern of fresh and cultured islets was qualitatively similar (Fig. 2F), in marked contrast with the secretion kinetics.
Similar metabolic and structural integrity of cultured and fresh islets
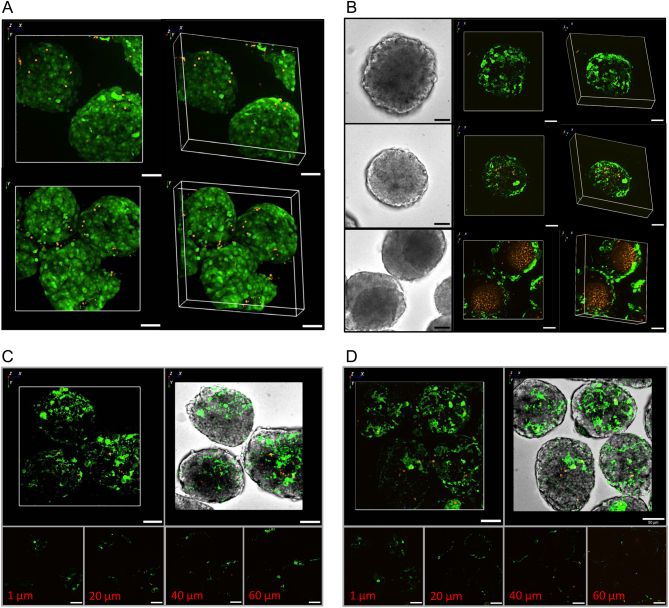
The generally higher prestimulatory [Ca2+]i levels in fresh islets gave rise to the concern that the secretion kinetics of these islets resulted from beta cell damage and that the more pronounced first phase was an expression of recovery by overnight culture. Therefore the viability of fresh and cultured islets was compared using the live/dead assay and imaging by spinning disk confocal microscopy. With both preparations the entirety of the islet was labelled by the calcein fluorescence, indicating that these cells had an intact plasma membrane. With both preparations fluorescent red dots, which indicate necrotic cells, were rare and confined to the islet periphery (Fig. 3A).
Figure 3.
Visualization of intact, apoptotic and necrotic cells in freshly isolated or cultured islets. (A) Freshly isolated (upper row) or 22-h-cultured (lower row) islets were loaded with calcein/AM and ethidium homodimer III. The red ethidium fluorescence of necrotic nuclei is occasionaly visible in the islet periphery, whereas the green fluorescence of the viability indicator calcein renders the entirety of the fresh and cultured islets visible. Representative images of five experiments. (B) Freshly isolated (upper row) or 22-h-cultured (middle row) islets were loaded with annexinV-Andy Fluor 488 to indicate incipient apoptosis and ethidium homodimer III to indicate necrotic nuclei. Beginning apoptosis is only visible in the outermost cell layer of fresh and cultured islets. Necrosis is negligible when compared to cultured islets showing a condensed core in transmitted light (lower row). Representative images of five experiments. (C and D) Fresh islets loaded with annexinV-Andy Fluor 488 and ethidium homodimer III were incubated for 60 min in Krebs–Ringer medium with 5 mM glucose (C) or 0 mM glucose (D); Shown is the epifluorescence (upper left) and the epifluorescence projected on the transmitted light image of the islets (upper right). Optical sections of the islets were made at depths of 1 µm (lower left), 20 µm (lower left middle), 40 µm (lower right middle) and 60 µm (lower right). Representative images of three experiments each.
Since the live/dead assay gives only a rather coarse view on the islet integrity and may not report on incipient damage, the possible onset of apoptosis was checked by the binding of fluorescently labelled annexin V (Fig. 3B). The binding of annexin V was confined to outermost cell layer of the fresh islets, in the same region red dots of ethidium binding were occasionally visible. The same pattern was seen with islets that had spent 22 h in culture under the same conditions as for functional testing. Islets which showed a dense core in transmitted light after culture were imaged as positive controls. While a thin mantle of annexin V-positive cells was also visible here, a massive accumulation of red fluorescent nuclei in the islet core demonstrated the extent of necrosis. Such extent of necrosis was never seen among the freshly isolated islets.
Finally, to test for the potentially damaging effect of the absence of nutrient in the perifusion medium for 1 h, the annexin V labeling was used to compare islets which had spent 1 h in the presence of 5 mM glucose with those which had spent the same time in the absence of glucose (Fig. 3C and D). A sketchy labeling in the outermost cell layer was visible after either treatment, but the islet core (which should be most sensitive to starvation) was practically unaffected.
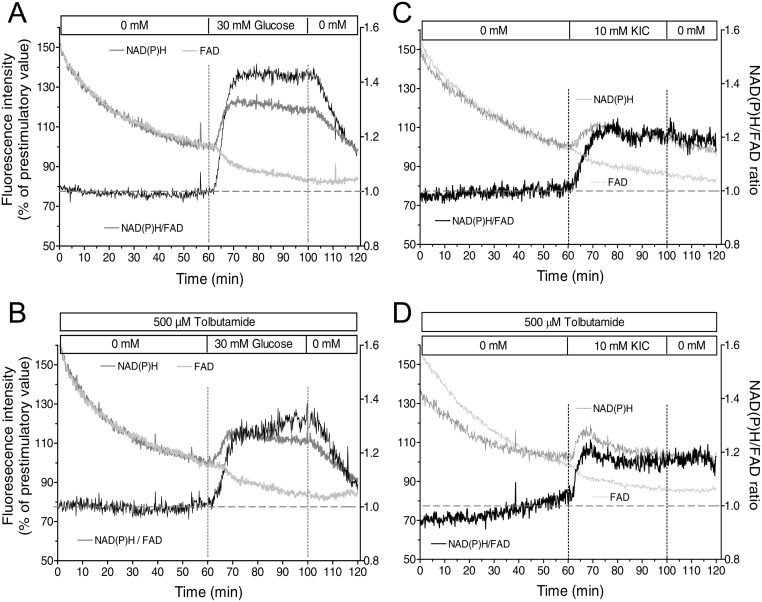
Kinetics of nutrient-generated reducing equivalents in cultured islets
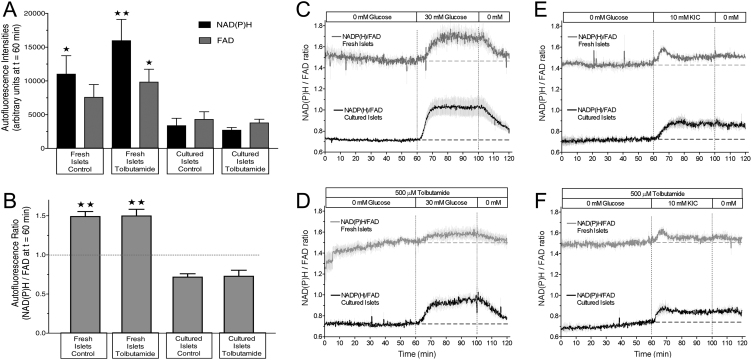
Since both triggering and amplifying signals emanate from the mitochondrial metabolism, the stimulation-induced changes of the NAD(P)H- and FAD-autofluorescence were measured under the same conditions as for the secretion measurements. The response to 30 mM glucose consisted in an increase of the NAD(P)H- and a slightly retarded decrease of the FAD-autofluorescence, both of which were promptly reversible upon washout. The specific increase in the levels of reducing equivalents can be highlighted by calculating the NAD(P)H/FAD ratio (Fig. 4A). The ratio showed a steady state followed by a steep increase during the first 10 min of glucose stimulation. Thereafter, an elevated plateau existed which decreased by 50% within 15 min upon wash-out. After tolbutamide-depolarization in the absence of exogenous nutrient glucose still elicited a qualitatively similar response, however the increase of the NAD(P)H/FAD ratio was smaller by about 30% (Fig. 4B). KIC produced an increase of the NAD(P)H/FAD-ratio which was smaller than the one by glucose by about 50% but which was practically unaffected by depolarization in the absence of nutrient (Fig. 4C and D). The kinetics of the KIC response was different from glucose in that an initial overshoot was followed by a moderately elevated plateau, which did not decrease during the wash-out phase.
Figure 4.
Increased levels of reducing equivalents by stimulation with nutrient secretagogues. 22-h-cultured isolated islets were perifused with Krebs–Ringer medium in the absence (A and C) or presence (B and D) of 500 µM tolbutamide. After perifusion without exogenous nutrient for 60 min either 30 mM glucose (A and B) or 10 mM KIC (C and D) was present from 60 to 100 min. From 100 to 120 min the nutrient stimuli were washed out. The fluorescence intensities were normalized to 100% at the last prestimulatory time point. The dark gray traces denote the NAD(P)H autofluorescence, the light gray traces the FAD autofluorescence and the black traces the NAD(P)H/FAD ratio as a semi-quantitative measure of mitochondrial reducing equivalents. Note the smaller and less reversible changes of the autofluorescences by KIC as compared with glucose. Values are means of 4–6 experiments each. s.e.m. ranges are omitted for clarity.
Kinetics of nutrient-generated reducing equivalents in cultured islets in comparison with those in fresh islets
Since the NAD(P)H- and FAD- fluorescence measurements were normalized to 100% with respect to the last prestimulatory value, a direct comparison with the normalized data of the earlier NAD(P)H- and FAD- measurements of fresh islets (16) could be misleading (Supplementary Fig. 2). Actually, the prestimulatory levels of the NAD(P)H- as well as the FAD-autofluorescence were two- to threefold lower in cultured islets than in fresh islets (Fig. 5A and Supplementary Fig. 3). Furthermore, the NAD(P)H-levels in cultured islets were more diminished than the FAD-levels, thus the prestimulatory NAD(P)H/FAD ratio in cultured islets was only about half as high as the one in fresh islets (0.75 vs 1.5 see Fig. 5B). These factors were used to make the NAD(P)H/FAD-ratios of cultured and fresh islets directly comparable (Fig. 5C, D, E and F). While the overall response pattern to nutrient stimulation was similar, for example, the response to glucose differed from that to KIC by the same criteria, the ratio values were constantly lower in the cultured islets. The most pronounced difference between cultured and fresh islets was visible when the glucose stimulus was applied in the presence of tolbutamide (Fig. 5D), which fits to the difference in secretion (Fig. 1B).
Figure 5.
Nutrient-induced increase of reducing equivalents in cultured islets as compared with freshly isolated islets. (A) Immediately prior to nutrient stimulation the NAD(P)H- and FAD-autofluorescence levels in cultured islets were significantly lower than in fresh islets (values taken from Schulze et al. 16). (B) At the same time point the NAD(P)H/FAD ratio in cultured islets was significantly lower than in fresh islets. Values are means of 4–6 experiments each. *P < 0.05, **P < 0.01 cultured vs fresh islets under the same condition. (C–F) These values were taken to enable an unbiased comparison of the NAD(P)H/FAD ratio kinetics of cultured islets (shown in Fig. 4) with those of freshly isolated islets. After perifusion without exogenous nutrient for 60 min either 30 mM glucose (C and D) or 10 mM KIC (E and F) was present from 60 to 100 min. In D and F 500 µM tolbutamide was continuously present. Values are means ± s.e.m. of 4–6 experiments each.
Different reactions of adenine nucleotide levels in cultured and fresh islets
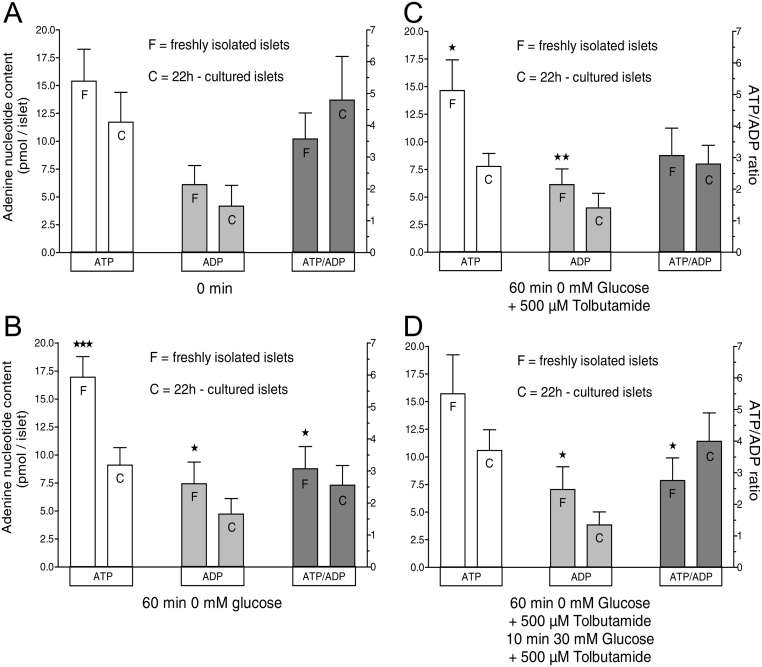
The contents of ATP and ADP of 22-h-cultured islets and freshly isolated islets were determined after static incubations designed to reflect four critical time points of the perifusion experiments: First, without incubation (Fig. 6A); second, 60 min in the absence of glucose or any other exogenous nutrient (Fig. 6B); third, 60 min without nutrient but with the additional presence of 500 µM tolbutamide (Fig. 6C); and finally (6D) 60 min as before plus 10 min exposure to 30 mM glucose in the continued presence of tolbutamide (see also the labels A-D in Fig. 1A and B). Without incubation neither ATP nor ADP nor the ATP/ADP ratio were significantly different, whereas after 60 min in the absence of exogenous nutrient all three parameters where higher in fresh islets. When tolbutamide was additionally present only ATP and ADP, but not the ATP/ADP ratio were higher in fresh islets. In this situation, the stimulation by glucose led to significantly higher levels of the ATP/ADP ratio in cultured islets, mainly due to lower levels of ADP. In short, the levels of adenine nucleotides are similar in cultured and fresh islets, but they evolve differently in response to nutrient deprivation and stimulation.
Figure 6.
Adenine nucleotide contents in cultured islets as compared with freshly isolated islets. (A–C) The contents of ATP and ADP of 22-h-cultured islets and freshly isolated islets were determined after static incubation in KR medium without glucose. The duration was 0 min (A), 60 min (B) or 60 min in the additional presence of 500 µM tolbutamide (C). (D) The fourth condition was 60 min followed by 30 mM glucose for 10 min, all in the additional presence of 500 µM tolbutamide. Values are means ± s.e.m. of six experiments each. *P < 0.05, **P < 0.01, ***P < 0.001, cultured vs fresh islets under the same condition, paired two-sided t-test.
Minor difference between cultured and fresh islets in responding to purely depolarizing stimuli
To examine whether cultured and fresh islets respond differently to purely depolarizing stimuli the prestimulatory concentration was set to 5 mM and the effects of 500 µM tolbutamide and of 40 mM KCl were compared (Fig. 7). With fresh islets tolbutamide and KCl both generated a secretion pattern with a predominant first phase and a plateau phase thereafter. Culturing did not appreciably affect the response to tolbutamide and moderately increased the plateau phase in the response to KCl (Fig. 7A and B). To test whether the prestimulatory glucose concentration of 5 mM was responsible for this result, the response to 30 mM glucose was tested as well. Here, a marked difference existed. After a comparable onset, the secretion of cultured islets decreased sharply and reached a nadir at 20 min, whereas the secretion of fresh islets only slowly decreased after a broad peak and resembled a square wave pattern (Fig. 7C). Both phases of secretion were significantly smaller in the cultured islets than in the fresh islets. This difference between cultured and fresh islets was also seen when the glucose concentration during culture was 10 mM (Fig. 7D).
Figure 7.
Kinetics of depolarization-induced insulin secretion of cultured and fresh islets. (A–C) 22-h-cultured islets (closed circles) or freshly isolated islets (open circles) were perifused with Krebs–Ringer medium containing 5 mM glucose for 60 min, then either 500 µM tolbutamide (A) or 40 mM KCl (B) or 30 mM glucose (C) was present for 40 min and was washed out thereafter. Note the similar secretion pattern of cultured and fresh islets in response to tolbutamide and KCl and the large difference in response to glucose. (D) This difference between cultured and fresh islets was also seen when the culture medium contained 10 mM glucose. Values are means ± s.e.m. of 4–5 experiments each. The inset shows the integrated insulin secretion from 60 to 75 min (1st phase) and from 60 min to washout (total). *P < 0.05, **P < 0.01, cultured vs fresh islets under the same condition, unpaired two-sided t-test.
Major but not exclusive role of FCS in shaping the predominant first phase kinetics of cultured islets
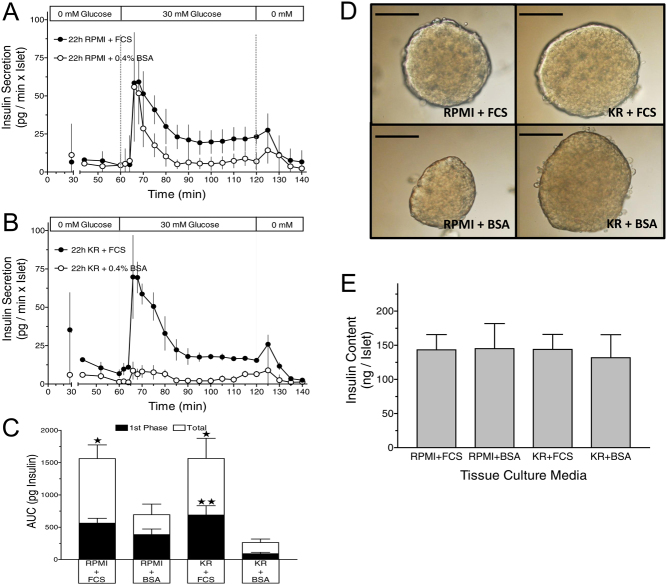
The contribution of the two main components of the cell culture medium, RPMI and FCS, to shape the kinetics of secretion was assessed by replacing RPMI with KR-medium and 10% (v/v) FCS with 0.4% (w/v) BSA during the culture period. The glucose concentration was always 5 mM. Even though the total mass of released insulin differed considerably, the rapid onset of the first phase was visible under all conditions (Fig. 8). Islets cultured in RPMI with BSA showed the same pattern of secretion in response to 30 mM glucose as the islets which had been cultured in RPMI with FCS (Fig. 8A). However, the second phase secretion plateau was significantly lower (Fig. 8C). Islets cultured in KR-medium with FCS produced a comparable secretion pattern (Fig. 8B). The first phase and total secretion were not significantly different from those after culture in RPMI with FCS, but differed significantly from those after culture in KR with BSA (Fig. 8C). Such islets responded to 30 mM glucose only with a modest increase for the first 15 min and a return to the very low prestimulatory levels thereafter. The different functional responses were not reflected by the appearance of the islets, which was essentially not different from islets after culture in RPMI plus FCS (Fig. 8D). Likewise, the total insulin content per islet was not significantly affected by the different culture conditions (Fig. 8E).
Figure 8.
Effect of the composition of the cell culture medium on the kinetics of insulin secretion. (A) Islets cultured for 22 h either in RPMI + 10% FCS (closed circles) or RPMI + 0.4% BSA (open circles) were perifused with Krebs–Ringer (KR) medium containing 0 mM glucose for 60 min, then 30 mM glucose was present for 60 min and was washed out thereafter. (B) The same protocol was used with islets cultured for 22 h either in KR + 10% FCS (closed circles) or KR + 0.4% BSA (open circles). (C) The amount of insulin released during the entire duration of glucose stimulation in (A) and (B) is shown by the white bars superimposed on the black bars, which show the one during the initial 15 min. *P < 0.05, **P < 0.01, when compared with the corresponding condition of BSA-cultured islets. Values are means ± s.e.m. of five experiments each. (D) The different functional responses after culture were not reflected the microscopic appearance of the islets. DIC contrast mode, the bar length is 100 µm. (E) The different culture conditions and secretion kinetics were not reflected by the insulin content of the islets. Values are means ± s.e.m. of five experiments each.
Discussion
The observations in this study suggest that the kinetics of insulin secretion which is regarded to be typical for mouse islets can be regularly observed after a culture phase, but much less frequently when freshly isolated islets are used. Cultured mouse islets nearly invariably responded to a square wave glucose stimulus with a prominent first phase secretion followed by a moderately elevated plateau. Depending on the prestimulatory conditions, the secretory response of fresh islets ranged from a moderate first phase followed by an ascending second phase to a virtual square wave response. In particular, the experimental condition reported to abolish the metabolic amplification of glucose, but not of KIC in fresh islets (14, 16) was unable to do so in cultured islets.
A cell culture period is widely considered to be essential for the work with isolated islets, since it is presumed to permit the recovery from the stress of collagenase isolation (17). It is also unavoidable if beta cells or islets are to be transfected. The more flexible experimental planning by the ready availability of cultured islets is an additional benefit. Currently, the wide availability of human islets (22) and the introduction of reconstituted islet spheroids (23) hold the promise to obtain more relevant information on human type 2 diabetes, but all these islets have spent considerable time under cell culture condition. The question is whether islet culture restores stimulus secretion coupling to its original state or leaves an imprint which may or may not correspond to the in vivo conditions.
It appears logical that islet culture diminishes the variability of the experimental observations seen with fresh islets, which reflects the different collagenase activities, the different methods of collagenase digestion (conventional method according to (24) vs duct injection according to (25)) and the different methods of islet collection (e.g. hand-picking vs density gradient collection, see (26)). Our experience shows that even minor changes in the collagenase isolation procedure can have detrimental consequences on the functional integrity of the freshly isolated islets. However, what precisely makes up the stress of isolation, which physiological functions of the islet are distorted by it and how culturing reverses these changes has not been well defined: Actually, much of the early ground-breaking work on the stimulus secretion coupling in beta cells was done with fresh islets (27).
The use of RPMI medium with the addition of 10% FCS was established early on as standard procedure for islet culture (28, 29, 30). Later, it was described that glucose at a moderate stimulatory concentration (11 mM) keeps the apoptosis rate during culture at a minimum (31), in line with the earlier observation that the absence of serum during culture could be partially compensated for by elevated glucose levels (29). Under our conditions necrotic cells were rare both in fresh and in 1-day-cultured islets. Apoptosis at an early stage was only visible in the outermost cell layer of fresh islets, which may mostly consist of exocrine cells. After the culture phase the labeling by annexin V was still visible but more scattered, suggesting some of these cells had disintegrated. The incubation of fresh islets for 60 min without exogenous nutrient did not increase the annexin V labeling and necrotic cells remained exceedingly rare. In our view fresh collagenase-isolated islets, if gently and quickly isolated (which is easier to achieve with mouse pancreas than with porcine or human pancreas) are structurally intact, establish steady state conditions within 1 h of perifusion and can give meaningful information on stimulus secretion coupling.
There are few publications addressing the secretion kinetics of cultured islets as compared with that of freshly isolated islets. Zawalich et al. found that a very short term culture (3 h) transformed the elevated plateau in response to 11 mM glucose into an ascending second phase (32). Ravier et al. described a strongly decreased steady state secretion, preceded by a predominant first phase, when mouse islets had been cultured overnight in 5 mM glucose (33). This change parallels our observation on how culturing transformed the secretion elicited by elevating glucose from 5 to 30 mM (Fig. 7C). The predominant first phase was also visible after overnight culture in 10 mM glucose even though the secretion level was markedly increased vs 5 mM (33).
In the intact organism a brisk first phase is particularly important for the glucose homeostasis (4, 5), so the more prominent first phase of cultured islets in response to glucose (Fig. 1A and B) may be viewed as a gain of functional competence. However, in response to KIC the freshly isolated islets showed the faster onset of secretion with a comparable magnitude of the first phase (Fig. 1C and D). This suggests that glucose-specific factors in stimulus-secretion coupling rather than unspecific structural defects were affected by the culture period. The close similarity between the kinetics of fresh and cultured islets when responding to purely depolarizing stimuli supports this view. Of note, microdissected islets showed a similar change of secretion kinetics as collagenase-isolated islets after culture (Fig. 2E), so the repair of collagenase-induced structural defects is unlikely to underlie the change in secretion kinetics.
Recently, the loss of the metabolic amplification by prior depolarization in the absence of exogenous nutrients was traced back to the time-dependent depletion of critical endogenous metabolites in freshly isolated islets (16, 34). Conversely, the preserved metabolic amplification in cultured islets would then be due to the higher abundance of endogenous metabolites or their slower consumption. In view of the lower prestimulatory NAD(P)H/FAD-ratios in the cultured islets the latter assumption may be correct. Here it has to be kept in mind that the level of reducing equivalents in the mitochondrial matrix, of which the NAD(P)H/FAD ratio is a semi-quantitative measure, is in flow equilibrium between generation in the Krebs cycle and consumption in the respiratory chain. Since we found cultured islets to consume oxygen at a lower rate than fresh islets (M Morsi and I Rustenbeck, unpublished observations), it may be the slower generation which is responsible for the lower level of reducing equivalents.
Both, the NAD(P)H- and the FAD-fluorescence levels were lower after culture. The contribution of NADPH to the NAD(P)H fluorescence signal is only of minor importance, since in beta cells glucose increases NADH as well as NADPH, probably coupled by a diminished NADPH consumption via the NNT (nicotinamide nucleotide transhydrogenase) running in the reverse direction (35). Interestingly, FAD was proportionally less decreased than NAD(P)H, resulting in the lower NAD(P)H/FAD ratio values (Fig. 5). Mitochondrial sites of FAD consumption are the succinate dehydrogenase reaction and the glycerol phosphate shuttle, where FADH2 is generated. Additionally, the dihydrolipoyl dehydrogenase within the pyruvate dehydrogenase complex reduces protein-bound FAD to FADH2 to ultimately generate NADH (for overviews on mitochondrial metabolism in beta cells see (36, 37, 38)).
A decreased activity of these enzymes, in particular of the pyruvate dehydrogenase offers a straightforward explanation for the decreased NAD(P)H/FAD ratio in cultured islets. It is conceivable that because of the resulting changes in mitochondrial metabolite flux the consumption of oxaloacetate is slower than in fresh islets, where oxaloacetate is likely the first Krebs cycle metabolite to become critically low during prolonged phases of depolarization in the absence of nutrients (15, 16, 34).
The adenine nucleotide levels were not significantly different in cultured and fresh islets at the beginning of the experiments. In view of the higher oxygen consumption of fresh islets mentioned above this may seem to indicate less well coupled mitochondria. However, the islet ATP content is strongly influenced by the ATP content in the insulin granules (39), which may obscure the relevant features. What appears to be relevant is different development of the adenine nucleotide contents during nutrient deprivation. Specifically, the lower content of adenine nucleotides and the lower ATP/ADP ratio in cultured islets after 60 min suggests a slower consumption of endogenous metabolites. The significantly higher ATP/ADP ratio in cultured islets after subsequent exposure to 30 mM glucose (Fig. 6D) together with a larger increase of the NAD(P)H/FAD ratio (Fig. 5D) suggests that under this condition glucose was better able to accelerate the oxidative phosphorylation, probably via the increased generation of reducing equivalents.
Another feature related to the different activity of oxidative phosphorylation is the marked transient decrease of [Ca2+]i in cultured islets at the beginning of the glucose stimulation, specifically when [Ca2+]i was elevated by prior exposure to tolbutamide (compare Fig. 2A and B). It is known that the transient dip is caused by the activation of SERCA pumps (40, 41), which respond to the ATP availability. The initial decrease in [Ca2+]i was found to be more pronounced after 1-day culture in 5 mM than after culture in 10 mM glucose (42). After culture in 10 mM glucose virtually no decrease in [Ca2+]i occurred, similar to the fresh islets in the present investigation. Thus the initial decrease is not an obligatory criterion for a functioning intracellular Ca2+ transport.
The generally higher prestimulatory [Ca2+]i levels in fresh islets were probably not caused by a lack of ATP required for Ca2+ sequestration and extrusion pumps, but may result from the reversible dissociation of gap junction channels (connexons) between beta cells whereby Ca2+-permeable hemichannels are formed (43, 44). While the initial kinetics of [Ca2+]i paralleled the increase of glucose-stimulated insulin secretion, the further course of secretion was not reflected by the changes in [Ca2+]i levels. This was as true for the cultured islets as for the fresh islets (Fig. 2A and C). So in both fresh and cultured islets the generation of amplifying signals appears to shape the secretion pattern.
Considering the consequences of exchanging the components of the culture medium (RPMI against KR and FCS against BSA) for the kinetics of glucose-induced secretion, it is obvious that the predominance of the first phase in cultured islets was a very robust feature (Fig. 8). So it is unlikely to result from specific nutrients contained in RPMI but not KR. FCS supported the overall secretory capacity much better than BSA, but its absence during culture did not abolish the predominant first phase. In this context we found the observation intriguing that mice in vivo were shown to have a biphasic response with an ascending second phase, which was no longer visible after culture of these islets (45). The authors hypothesized that the difference was due to the loss of an in vivo input like innervation or incretin hormones. The fact that the secretion pattern of the perfused mouse pancreas (46, 47) is similar to that of cultured islets also points in this direction.
In summary, the secretion kinetics of fresh mouse islets is more responsive to variations of nutrient stimulation whereas cultured islets respond with uniform kinetics, the pattern of which resembles the one elicited by purely depolarizing stimuli. Even though the isolation procedure may affect islet function, it is not damaging to the islet structure, if appropriately performed. Conversely, the altered kinetics after culture does not simply represent a reset to the original working conditions. It remains to be clarified whether it is due to a lack of signals present in vivo or whether general conditions of the culture phase leave an imprint on the nutrient metabolism of the beta cell.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG Ru 368/5-4) and the DDG – Deutsche Diabetes Gesellschaft. T S was supported by the Quanomet Project of the Lower Saxony Ministry for Science and Culture.
Author contribution statement
M M isolated the pancreatic islets and performed microfluorometric and insulin secretion experiments. T S and E F isolated the pancreatic islets and measured the cytosolic Ca2+ concentration. D B performed imaging. I R and U P conceived experiments and wrote the manuscript. All authors discussed results.
Acknowledgements
Expert technical assistance by Angela Hahlbohm, Verena Lier-Glaubitz and Johannes Sievers (Institute of Pharmacology, Toxicology and Clinical Pharmacy, Technische Universität Braunschweig) is gratefully acknowledged.
References
- 1.Nesher R, Cerasi E. Biphasic insulin release as the expression of combined inhibitory and potentiating effects of glucose. Endocrinology 1987. 121 1017–1024. ( 10.1210/endo-121-3-1017) [DOI] [PubMed] [Google Scholar]
- 2.Nesher R, Cerasi E. Modeling phasic insulin release: immediate and time-dependent effects of glucose. Diabetes 2002. 51 (Supplement 1) S53–S59. ( 10.2337/diabetes.51.2007.s53) [DOI] [PubMed] [Google Scholar]
- 3.Cerasi E, Luft R, Efendic S. Decreased sensitivity of the pancreatic beta cells to glucose in prediabetic and diabetic subjects. A glucose dose-response study. Diabetes 1972. 21 224–234. ( 10.2337/diab.21.4.224) [DOI] [PubMed] [Google Scholar]
- 4.Gerich JE. Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes 2002. 51 (Supplement 1) S117–S121. ( 10.2337/diabetes.51.2007.s117) [DOI] [PubMed] [Google Scholar]
- 5.Del Prato S. Loss of early insulin secretion leads to postprandial hyperglycaemia. Diabetologia 2003. 46 (Supplement 1) M2–M8. ( 10.1007/s00125-002-0930-6) [DOI] [PubMed] [Google Scholar]
- 6.Rorsman P, Renström E. Insulin granule dynamics in pancreatic beta cells. Diabetologia 2003. 46 1029–1045. ( 10.1007/s00125-003-1153-1) [DOI] [PubMed] [Google Scholar]
- 7.Straub SG, Sharp GW. Hypothesis: one rate-limiting step controls the magnitude of both phases of glucose-stimulated insulin secretion. American Journal of Physiology: Cell Physiology 2004. 287 C565–C571. ( 10.1152/ajpcell.00079.2004) [DOI] [PubMed] [Google Scholar]
- 8.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000. 49 1751–1760. ( 10.2337/diabetes.49.11.1751) [DOI] [PubMed] [Google Scholar]
- 9.Daniel S, Noda M, Straub SG, Sharp GW. Identification of the docked granule pool responsible for the first phase of glucose-stimulated insulin secretion. Diabetes 1999. 48 1686–1690. ( 10.2337/diabetes.48.9.1686) [DOI] [PubMed] [Google Scholar]
- 10.Henquin JC, Nenquin M, Stiernet P, Ahren B. In vivo and in vitro glucose-induced biphasic insulin secretion in the mouse: pattern and role of cytoplasmic Ca2+ and amplification signals in beta-cells. Diabetes 2006. 55 441–451. ( 10.2337/diabetes.55.02.06.db05-1051) [DOI] [PubMed] [Google Scholar]
- 11.Hatlapatka K, Willenborg M, Rustenbeck I. Plasma membrane depolarization as a determinant of the first phase of insulin secretion. American Journal of Physiology: Endocrinology and Metabolism 2009. 297 E315–E322. ( 10.1152/ajpendo.90981.2008) [DOI] [PubMed] [Google Scholar]
- 12.Panten U, Schwanstecher M, Wallasch A, Lenzen S. Glucose both inhibits and stimulates insulin secretion from isolated pancreatic islets exposed to maximally effective concentrations of sulfonylureas. Naunyn-Schmiedeberg’s Archives of Pharmacology 1988. 338 459–462. ( 10.1007/BF00172128) [DOI] [PubMed] [Google Scholar]
- 13.Gembal M, Gilon P, Henquin JC. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. Journal of Clinical Investigation 1992. 89 1288–1295. ( 10.1172/JCI115714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urban KA, Panten U. Selective loss of glucose-induced amplification of insulin secretion in mouse pancreatic islets pretreated with sulfonylurea in the absence of fuels. Diabetologia 2005. 48 2563–2566. ( 10.1007/s00125-005-0030-5) [DOI] [PubMed] [Google Scholar]
- 15.Panten U, Willenborg M, Schumacher K, Hamada A, Ghaly H, Rustenbeck I. Acute metabolic amplification of insulin secretion in mouse islets is mediated by mitochondrial export of metabolites, but not by mitochondrial energy generation. Metabolism: Clinical and Experimental 2013. 62 1375–1386. ( 10.1016/j.metabol.2013.05.006) [DOI] [PubMed] [Google Scholar]
- 16.Schulze T, Morsi M, Reckers K, Brüning D, Seemann N, Panten U, Rustenbeck I. Metabolic amplification of insulin secretion is differentially desensitized by depolarization in the absence of exogenous fuels. Metabolism: Clinical and Experimental 2017. 67 1–13. ( 10.1016/j.metabol.2016.10.008) [DOI] [PubMed] [Google Scholar]
- 17.Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biological Procedures Online 2009. 11 3–31. ( 10.1007/s12575-009-9021-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willenborg M, Schumacher K, Rustenbeck I. Determination of beta-cell function: insulin secretion of isolated islets. Methods in Molecular Biology 2012. 933 189–201. ( 10.1007/978-1-62703-068-7_12) [DOI] [PubMed] [Google Scholar]
- 19.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. Journal of Experimental Medicine 1995. 182 1545–1556. ( 10.1084/jem.182.5.1545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panten U, Ishida H. Fluorescence of oxidized flavoproteins from perifused isolated pancreatic islets. Diabetologia 1975. 11 569–573. ( 10.1007/BF01222108) [DOI] [PubMed] [Google Scholar]
- 21.Rocheleau JV, Head WS, Piston DW. Quantitative NAD(P)H/flavoprotein autofluorescence imaging reveals metabolic mechanisms of pancreatic islet pyruvate response. Journal of Biological Chemistry 2004. 279 31780–31787. ( 10.1074/jbc.M314005200) [DOI] [PubMed] [Google Scholar]
- 22.Henquin JC. The challenge of correctly reporting hormones content and secretion in isolated human islets. Molecular Metabolism 2019. 30 230–239. ( 10.1016/j.molmet.2019.10.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HJ, Alam Z, Hwang JW, Hwang YH, Kim MJ, Yoon S, Byun Y, Lee DY. Optimal formation of genetically modified and functional pancreatic islet spheroids by using hanging-drop strategy. Transplantation Proceedings 2013. 45 605–610. ( 10.1016/j.transproceed.2012.11.014) [DOI] [PubMed] [Google Scholar]
- 24.Moskalewski S. Isolation and culture of the islets of Langerhans of the guinea pig. General and Comparative Endocrinology 1965. 5 342–353. ( 10.1016/0016-6480(65)90059-6) [DOI] [PubMed] [Google Scholar]
- 25.Gotoh M, Maki T, Satomi S, Porter J, Bonner-Weir S, O'Hara CJ, Monaco AP. Reproducible high yield of rat islets by stationary in vitro digestion following pancreatic ductal or portal venous collagenase injection. Transplantation 1987. 43 725–730. ( 10.1097/00007890-198705000-00024) [DOI] [PubMed] [Google Scholar]
- 26.Brunstedt J. Rapid isolation of functionally intact pancreatic islets from mice and rats by Percoll gradient centrifugation. Diabete and Metabolisme 1980. 6 87–89. [PubMed] [Google Scholar]
- 27.Hedeskov CJ. Mechanism of glucose-induced insulin secretion. Physiological Reviews 1980. 60 442–509. ( 10.1152/physrev.1980.60.2.442) [DOI] [PubMed] [Google Scholar]
- 28.Buitrago A, Gylfe E, Hellman B, Idahl LA, Johansson M. Function of microdissected pancreatic islets cultured in a chemically defined medium. I. Insulin content and release. Diabetologia 1975. 11 535–540. ( 10.1007/BF01222103) [DOI] [PubMed] [Google Scholar]
- 29.Andersson A. Isolated mouse pancreatic islets in culture: effects of serum and different culture media on the insulin production of the islets. Diabetologia 1978. 14 397–404. ( 10.1007/BF01228134) [DOI] [PubMed] [Google Scholar]
- 30.Andersson A, Borg H, Hallberg A, Hellerström C, Sandler S, Schnell A. Long-term effects of cyclosporin A on cultured mouse pancreatic islets. Diabetologia 1984. 27 (Supplement) 66–69. ( 10.1007/BF00275649) [DOI] [PubMed] [Google Scholar]
- 31.Efanova IB, Zaitsev SV, Zhivotovsky B, Köhler M, Efendić S, Orrenius S, Berggren PO. Glucose and tolbutamide induce apoptosis in pancreatic beta-cells. A process dependent on intracellular Ca2+ concentration. Journal of Biological Chemistry 1998. 273 33501–33507. ( 10.1074/jbc.273.50.33501) [DOI] [PubMed] [Google Scholar]
- 32.Zawalich WS, Yamazaki H, Zawalich KC. Biphasic insulin secretion from freshly isolated or cultured, perifused rodent islets: comparative studies with rats and mice. Metabolism: Clinical and Experimental 2008. 57 30–39. ( 10.1016/j.metabol.2007.07.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravier MA, Nenquin M, Miki T, Seino S, Henquin JC. Glucose controls cytosolic Ca2+ and insulin secretion in mouse islets lacking adenosine triphosphate-sensitive K+ channels owing to a knockout of the pore-forming subunit Kir6.2. Endocrinology 2009. 150 33–45. ( 10.1210/en.2008-0617) [DOI] [PubMed] [Google Scholar]
- 34.Panten U, Früh E, Reckers K, Rustenbeck I. Acute metabolic amplification of insulin secretion in mouse islets: role of cytosolic acetyl-CoA. Metabolism: Clinical and Experimental 2016. 65 1225–1229. ( 10.1016/j.metabol.2016.05.001) [DOI] [PubMed] [Google Scholar]
- 35.Santos LRB, Muller C, de Souza AH, Takahashi HK, Spégel P, Sweet IR, Chae H, Mulder H, Jonas JC. NNT reverse mode of operation mediates glucose control of mitochondrial NADPH and glutathione redox state in mouse pancreatic β-cells. Molecular Metabolism 2017. 6 535–547. ( 10.1016/j.molmet.2017.04.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jitrapakdee S, Wutthisathapornchai A, Wallace JC, MacDonald MJ. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia 2010. 53 1019–1032. ( 10.1007/s00125-010-1685-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholls DG. The pancreatic β-cell: a bioenergetic perspective. Physiological Reviews 2016. 96 1385–1447. ( 10.1152/physrev.00009.2016) [DOI] [PubMed] [Google Scholar]
- 38.Fex M, Nicholas LM, Vishnu N, Medina A, Sharoyko VV, Nicholls DG, Spégel P, Mulder H. The pathogenetic role of β-cell mitochondria in type 2 diabetes. Journal of Endocrinology 2018. 236 R145–R159. ( 10.1530/JOE-17-0367) [DOI] [PubMed] [Google Scholar]
- 39.Detimary P, Jonas JC, Henquin JC. Stable and diffusible pools of nucleotides in pancreatic islet cells. Endocrinology 1996. 137 4671–4676. ( 10.1210/endo.137.11.8895332) [DOI] [PubMed] [Google Scholar]
- 40.Chow RH, Lund PE, Löser S, Panten U, Gylfe E. Coincidence of early glucose-induced depolarization with lowering of cytoplasmic Ca2+ in mouse pancreatic beta-cells. Journal of Physiology 1995. 485 607–617. ( 10.1113/jphysiol.1995.sp020756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hellman B, Grapengiesser E. Glucose-induced inhibition of insulin secretion. Acta Physiologica 2014. 210 479–488. ( 10.1111/apha.12217) [DOI] [PubMed] [Google Scholar]
- 42.Gilon P, Jonas JC, Henquin JC. Culture duration and conditions affect the oscillations of cytoplasmic calcium concentration induced by glucose in mouse pancreatic islets. Diabetologia 1994. 37 1007–1014. ( 10.1007/BF00400464) [DOI] [PubMed] [Google Scholar]
- 43.Ravier MA, Güldenagel M, Charollais A, Gjinovci A, Caille D, Söhl G, Wollheim CB, Willecke K, Henquin JC, Meda P. Loss of connexin36 channels alters beta-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes 2005. 54 1798–1807. ( 10.2337/diabetes.54.6.1798) [DOI] [PubMed] [Google Scholar]
- 44.Seemann N, Welling A, Rustenbeck I. The inhibitor of connexin Cx36 channels, mefloquine, inhibits voltage-dependent Ca2+ channels and insulin secretion. Molecular and Cellular Endocrinology 2018. 472 97–106. ( 10.1016/j.mce.2017.11.024) [DOI] [PubMed] [Google Scholar]
- 45.Nunemaker CS, Wasserman DH, McGuinness OP, Sweet IR, Teague JC, Satin LS. Insulin secretion in the conscious mouse is biphasic and pulsatile. American Journal of Physiology: Endocrinology and Metabolism 2006. 290 E523–E529. ( 10.1152/ajpendo.00392.2005) [DOI] [PubMed] [Google Scholar]
- 46.Lenzen S. Insulin secretion by isolated perfused rat and mouse pancreas. American Journal of Physiology 1979. 236 E391–E400. ( 10.1152/ajpendo.1979.236.4.E391) [DOI] [PubMed] [Google Scholar]
- 47.Berglund O. Different dynamics of insulin secretion in the perfused pancreas of mouse and rat. Acta Endocrinologica 1980. 93 54–60. ( 10.1530/acta.0.0930054) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a