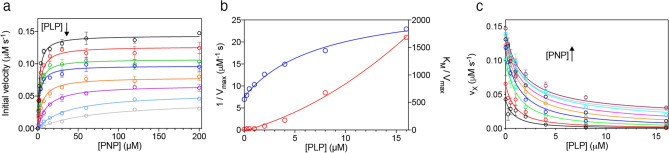
Figure 4.
Characterization of PLP inhibition. (a) The initial velocity of the reaction was measured with 2 µM enzyme (protein subunit concentration), varying PNP concentration while keeping exogenous PLP fixed at different concentrations (0, 0.25, 0.5, 1, 2, 4, 8 and 16 µM, as indicated by the pointing down arrow). Data are the average ± standard deviation of three independent measurements. The Michaelis–Menten equation was fitted to the resulting saturation curves, obtaining apparent Vmax and KM values at all PLP concentrations. (b) Fitting of 1/Vmax (blue symbols) and KM/Vmax (red symbols), obtained from the fitting of data shown in panel A, using an increasing hyperbolic equation and a parabolic equation, respectively. (c) Initial velocity data rearranged as a function of PLP concentration, and obtained at different, fixed substrate concentrations (vX). The increasing PNP concentration is indicated by the pointing up arrow. Continuous lines result from global fitting of vx values to Eq. (3), as detailed in the Methods section, which gave the parameters reported in the text. Images were generated using the software Prism 8 (GraphPad; https://www.graphpad.com/scientific-software/prism/).