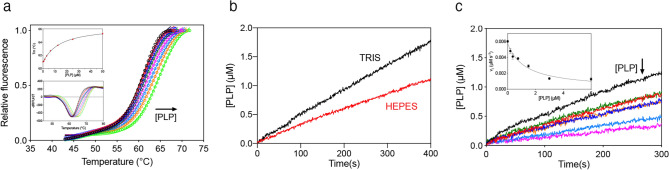
Figure 7.
PLP binding and allosteric properties of the PNPO R225H variant. (a) Fluorescence change expressed as fractional variation is plotted as a function of temperature. The experimental conditions were the same as those reported in Fig. 6c. The curves are the result of three independent experiments but, to better show the data, the standard error bars were not reported in the graph. In the upper inset, the saturation curve obtained by plotting the melting temperatures as a function of the PLP concentrations is shown. Data were analysed using the quadratic Eq. (4) (see Methods) to estimate the dissociation constant reported in Table 1. In the lower inset, first derivative (–dRFU/dT) of the same data reported in panel A. Kinetic studies of R225H variant. (b) Comparison of kinetics carried out in 50 mM TRIS–HCl and 50 mM Na-HEPES buffers at pH 7.6, obtained using 0.5 μM enzyme and 60 μM PNP. (c) Kinetics obtained by the addition of increasing concentrations of exogenous PLP (0, 0.19, 0.38, 0.75, 1.5, 3 and 6 µM, as indicated by the pointing down arrow) to reactions. The experiments were carried out with 1 μM enzyme and 60 μM PNP. A decreasing hyperbolic equation was fitted to the initial velocity of the reaction as a function of PLP concentration (inset). Images were generated using the software Prism 8 (GraphPad; https://www.graphpad.com/scientific-software/prism/).