Abstract
Background
Desmoplastic small round cell tumor (DSRCT) is a rare intra-abdominal soft tissue sarcoma affecting adolescents and young adults. Cytoreduction, hyperthermic intraperitoneal chemotherapy (CRS/HIPEC), and adjuvant radiotherapy may improve local control. We review our experience with patients who undergo CRS/HIPEC and adjuvant radiotherapy for DSRCT.
Methods
A retrospective review was performed for patients with DSRCT from 2013–2017 who underwent CRS/HIPEC. Clinicopathologic, operative and outcome data were reviewed.
Results
Ten CRS/HIPEC procedures were performed for 9 patients (7 male, 6 Caucasian, median age 19 years (range, 10–24)). Four patients presented with extra-abdominal disease; 5 had liver involvement. The median peritoneal cancer index was 16 (range, 5–20). All received neoadjuvant chemotherapy. CCR 0/1 resection was possible in 9 patients. Major complications occurred in 4 with no operative mortalities. All received adjuvant chemotherapy, seven received radiation therapy, and three received stem cell transplant. All but one patient recurred after treatment. The median recurrence free and overall survival (OS) were 12 and 45 months (95% CI 35.1–54.9) respectively, with a 3-year OS of 55%. Long-term parenteral nutrition was required in 8 for a median of 261 days (range, 37–997). Clinically significant long-term complications requiring further surgery included gastroparesis (N=1), small bowel obstruction (N=3) and hemorrhagic cystitis (N=2).
Conclusion
Multimodal therapy for DSRCT consisting of multi-agent neoadjuvant chemotherapy, CRS/HIPEC, adjuvant chemotherapy and radiation therapy is associated with potential cumulative toxicity. Recurrence after resection is common. Prolonged parenteral nutrition may be necessary and late gastrointestinal and genitourinary complications may require additional treatment.
Keywords: Carcinomatosis, Cytoreduction, Hyperthermic Intraperitoneal Chemotherapy, DSRCT, Sarcomatosis
INTRODUCTION
Desmoplastic small round cell tumor (DSRCT) is a rare and highly aggressive mesenchymal tumor that occurs in adolescents and young adults.[1] Males are more frequently affected than females with a peak age of onset of 20–24 years.[2] DSRCT most often originates in the abdominal cavity and presents with tumors lining the serosal surfaces of the abdomen and pelvis (sarcomatosis).[3] For this reason, complete surgical resection at initial diagnosis is often not possible.
The optimal treatment paradigm for DSRCT remains poorly defined. Surgical resection is the mainstay of therapy and debulking of more than 90% of the tumor burden coupled with a response to multi-agent chemotherapy is associated with improved survival.[4] While DSRCT is chemosensitive, recurrence after resection is common.[5] An aggressive multimodality treatment approach consisting of high-dose, multi-agent chemotherapy (the P6 protocol), surgery and radiation therapy was the first treatment strategy to demonstrate improved survival. [6, 7] Furthermore, while limited-field radiotherapy has been suggested to be inferior to whole abdominal radiotherapy (WART), the optimal radiotherapy dose and treatment volume remain poorly defined.[5, 8, 9]
Cytoreduction with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) has emerged as a feasible treatment for abdominal pediatric sarcomatosis with acceptable perioperative morbidity.[10, 11] Based on initial reports of feasibility and potential impact on local control success, our center began to perform CRS/HIPEC in 2013 as a component of the management of DSRCT.[12–15] However, the long-term impact of aggressive multimodality therapy for DSRCT remains poorly defined. We herein report our single-institutional outcomes for DSRCT after CRS/HIPEC and describe long-term complications related to the cumulative toxicity of multimodality therapy.
METHODS
Patient Selection
We performed a retrospective review of patients who underwent CRS/HIPEC for DSRCT from January 2013 to December 2017 at St Jude Children’s Research Hospital. All patients were discussed in multidisciplinary tumor board prior to consideration for CRS/HIPEC. Patients with disease outside of the abdominal cavity were considered for CRS/HIPEC if the disease was responsive to neoadjuvant chemotherapy and resolved on PET/CT imaging. All patients underwent surgery with intent of complete cytoreduction. Approval for this study was obtained from the University of Tennessee Health Science Center and St Jude Children’s Research Hospital institutional review boards.
Cytoreduction/HIPEC
The peritoneal cancer index (PCI) score was calculated at the time of surgical exploration.[16] HIPEC was performed via a closed technique at the completion of cytoreduction. HIPEC was performed with mitomycin-C (40 mg at 42°C for 90 minutes, N=1), melphalan (50 mg for 90 minutes, N=1) or cisplatin (100 mg/m2 at 42°C for 60 minutes, N=8). The adequacy of surgical debulking was defined by the completeness of cytoreduction (CCR) score.[17] Cytoreduction was considered CCR 0 if no visible disease remained after surgery, CCR-1 if disease less than 0.25 cm, CCR-2 if disease was greater than 0.25 cm but less than 2.5 cm and CCR-3 if disease was greater than 2.5 cm.
Outcome
Outcomes of interest included length of hospital stay, treatment related morbidity, need for post-operative nutritional support, and salvage therapy including additional procedures. Surgical complications were graded according to the Clavien-Dindo classification schema.[18] Recurrence was defined as radiographic evidence (PET/CT, MRI, Ultrasound, and/or CT) of disease any time after CRS/HIPEC. Recurrence free survival (RFS) was defined as the time from CRS/HIPEC to development of disease recurrence. Overall survival (OS) was defined as the time from CRS/HIPEC to death or censored at the date of last follow up. Patients were stratified based on DSRCT clinical stage with Stage 1-PCI < 12, Stage II-PCI>12, Stage III-Any PCI with presence of liver metastasis and Stage IV-Any PCI with presence of extra-abdominal metastasis.[10]
Statistical Analysis
For all outcomes, patients were censored at the time of most recent follow-up or death. Categorical variables were summarized using percentages while continuous variables were summarized using median and range. RFS and OS were summarized using the Kaplan-Meier method and compared across strata using the log rank test. All statistical analysis was performed using SPSS software, version 24 (IBM Corporation, Armonk, NY).
RESULTS
Patient Demographics
Nine patients underwent 10 CRS/HIPEC procedures for DSRCT (one patient had repeat HIPEC for intra-abdominal recurrence after being disease-free for more than 30 months from initial HIPEC). The median patient age was 19 years (range, 10–24 years), 6 were Caucasian and 7 were male. At initial presentation, evidence of liver disease was present in 5 patients, 4 patients had extra-abdominal disease (all with mediastinal lymphadenopathy), and 6 patients had intra-abdominal nodal disease. All patients received neoadjuvant chemotherapy with a median time from diagnosis of DSRCT to CRS/HIPEC of 7 months (range, 2–38 months). Given the referral pattern of our patient population, the median distance travelled to receive treatment and undergo CRS/HIPEC was 767 miles (range, 148–2081 miles).
Cytoreduction/HIPEC
The median PCI score was 16 (range, 5–20). Multivisceral resection was performed in 9/10 CRS/HIPEC procedures with 6 patients undergoing resection of 4 or more organs. Ureteral catheters were placed in all patients at the time of surgery. Omentectomy was the most common procedure performed, followed by rectal resection. Pelvic peritonectomy was performed in 9 patients and right diaphragm peritonectomy in 7. A CCR-0 resection was achieved in 5, CCR-1 in 4 patients and CCR-2 in 1 patient. A detailed description of operative variables and procedures performed is shown in Table 1.
Table 1.
Operative characteristics and procedures performed for 9 patients who underwent 10 CRS/HIPEC procedures for DSRCT.
| Variable | N (%) |
|---|---|
| Peritoneal Cancer Index | 16 (5–20) |
| Cytoreduction Score (CCR) | |
| CCR-0 | 5 (50) |
| CCR-1 | 4 (40) |
| CCR-2 | 1 (10) |
| Length of Surgery (minutes) | 551 (410–725) |
| Estimated Blood Loss (mL) | 900 (300–4300) |
| Multi-visceral Resection | 9 (90) |
| ≥ 4 organs | 6 (60) |
| Any Anastomosis | 5 (50) |
| One | 4 (80) |
| Two | 1 (20) |
| Ostomy Creation | 5 (50) |
| Diverting Ileostomy | 4 (80) |
| End Colostomy | 1(20) |
| Procedure Description | |
| Ureteral Catheter Placement | 10 (100) |
| Omentectomy | 9 (90) |
| Rectal Resection | 6 (60) |
| Cholecystectomy | 7 (70) |
| Appendectomy | 6 (60) |
| Ureterolysis | 5 (50) |
| Tube Thoracostomy | 4 (40) |
| Liver Resection | 3 (30) |
| Gastric Resection | 2 (20) |
| Pancreatectomy | 1 (10) |
| Colectomy | 1 (10) |
| Splenectomy | 1 (10) |
| Hysterectomy | 1 (10) |
| Oophorectomy | 1 (10) |
| Parietal Peritonectomy | |
| Pelvic | 9 (90) |
| Diaphragm | 7 (70) |
| Flank | 3 (30) |
| Right Lower Quadrant | 3 (30) |
| Left Lower Quadrant | 3 (30) |
| Lymph Node Dissection | |
| Pelvic | 3 (30) |
| Iliac | 2 (20) |
| Aortocaval | 2 (20) |
| Porto Hepatis | 1 (10) |
| Celiac | 1 (10) |
| Superior Mesenteric Artery | 1 (10) |
Results presented as Frequency (Percentage) or Median (Range).
Perioperative Outcome
All patients were managed in the intensive care unit (ICU) post-operatively (Table 2).Five patients required post-operative mechanical ventilation and underwent extubation the following day. Minor morbidity (Clavien-Dindo 1/2) was observed in 5 patients: wound infection (3), ileus (1) and urinary retention/urinary tract infection (1). Four patients experienced a major complication (Clavien-Dindo 3/4): pneumothorax (1), pelvic abscess (1), AKI/pelvic abscess/anastomotic dehiscence (1), AKI/hemodialysis/respiratory failure/pelvic abscess (1). There were no operative or 30-day mortalities.
Table 2.
Perioperative characteristics, morbidity, adjuvant treatment and outcome after CRS/HIPEC for DSRCT.
| Variable | N (%) |
|---|---|
| ICU Length of Stay (days) | 3 (1–25) |
| Hospital Length of Stay (days) | 9 (8–38) |
| Complication-Minor | 5 (56) |
| Wound Infection | 3 |
| Ileus | 1 |
| Urinary Retention/Urinary Tract Infection | 1 |
| Complication-Major | 4 (44) |
| Pneumothorax | 1 |
| Pelvic Abscess | 1 |
| AKI/Pelvic Abscess/Anastomotic Dehiscence | 1 |
| AKI/Hemodialysis/Respiratory Failure/Pelvic Abscess | 1 |
| Mortality (30 day) | 0 |
| Whole Abdomen Radiation Therapy | 7 (78) |
| Stem Cell Transplant | 3 (33) |
| Adjuvant Chemotherapy | 8 (89) |
| Recurrence | 8 (89) |
| Abdominal | |
| Liver/Peritoneum | 3 |
| Liver Only | 1 |
| Peritoneum Only | 1 |
| Extra-abdominal | |
| R Tibia | 1 |
| Retroperitoneal LN | 1 |
| Lung | 1 |
| Time to Recurrence (months) | 17 (10–48) |
| Follow Up (months) | 34.0 (17–58) |
Results presented as Frequency (Percentage) or Median (Range)
Patterns of Failure and Survival after CRS/HIPEC
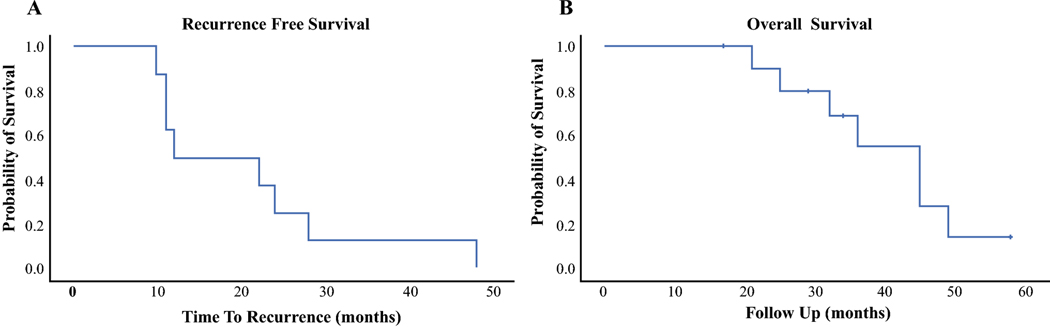
Adjuvant chemotherapy was administered to all patients after CRS/HIPEC. Whole abdomen radiation therapy (WART) was administered to 7 patients with 3 patients undergoing stem cell transplant. All but one patient recurred after CRS/HIPEC; median time to recurrence was 17 months (range, 10–48). Five patients recurred within the abdominal cavity while 3 patients developed extra-abdominal recurrence. No patient recurred in fields receiving a high dose radiotherapy volume (>50 Gy). The most frequent area of intra-abdominal recurrence within the WART field (>30 Gy) was the peri-diaphragmatic region. The most common non-peritoneal site of recurrence was intrahepatic. Of the 4 patients who presented with liver metastasis, 3 recurred within the liver as a component of failure. Estimated median RFS was 12 months and the 1-year and 3-year RFS were 50% and 13%, respectively (Figure 1A). The median OS was 45 months and the 1-year and 3-year OS were 100% and 55%, respectively (Figure 1B). With a median follow up of 34 months (range, 17–58), 2 patients were alive with disease while the remainder had died of disease (Table 2).
Figure 1A-B:
Recurrence free survival (A) and overall survival (B) after CRS/HIPEC for DSRCT
Prognostic Factors
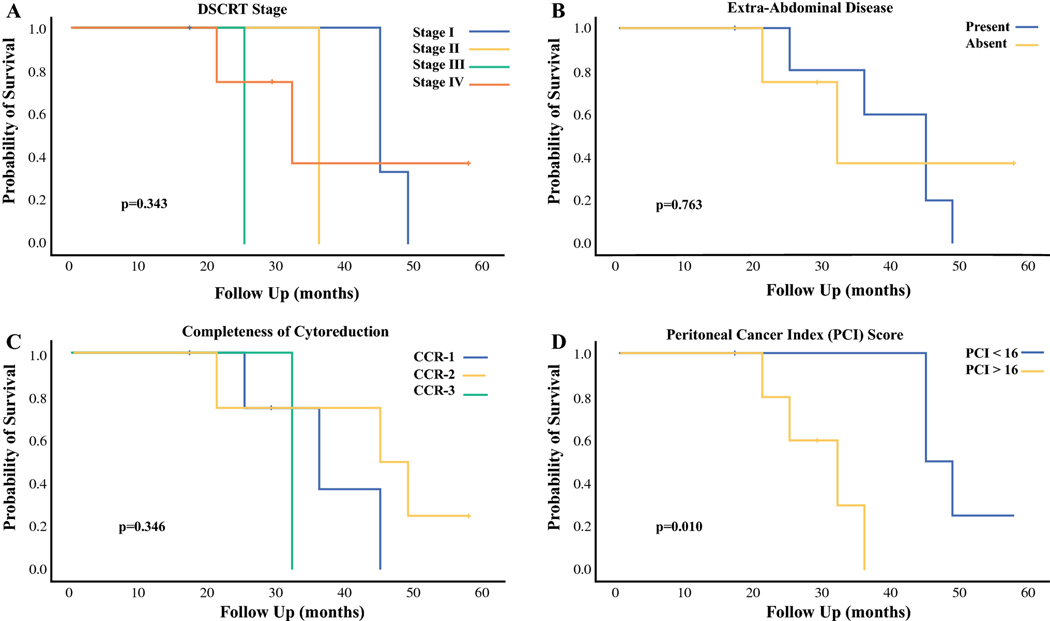
There was no difference in median OS based on DSRCT clinical stage (Stage I-45 months, Stage II-36 months, Stage III-25 months and Stage IV-32 months, p=0.343, Figure 2A). The presence of extra-abdominal disease at initial presentation did not have a significant impact on OS (45 months, 95% CI 37–53 months versus 32 months, 95% CI 16–48 months, p=0.763, Figure 2B). Similarly, there was no significant difference in OS based on the presence of disease within the liver, intra-abdominal nodal disease, or patient age (data not shown). The median OS was 36 months (95% CI 20–52 months) for those who underwent a CCR-0 resection, 45 months (95% CI 18–72 months) for those who underwent a CCR-1 resection and 32 months for the single patient who underwent a CCR-2 resection (p=0.346, Figure 2C). Patients with a PCI < 16 had a better OS than those with a PCI > 16 (45 months versus 32 months, p=0.010, Figure 2D).
Figure 2A-D:
Overall survival after CRS/HIPEC based on DSRCT clinical stage (A), presence of extra-abdominal disease (B), completeness of cytoreduction (C) and peritoneal cancer index (PCI) score (D).
Long-Term Complications and Subsequent Procedures
Additional hospitalization was required in 8 of the 9 patients for medical and surgical-related indications. The median number of additional hospital days was 76 days (range 32–298 days). Long-term parenteral nutrition was required in 8 patients for a median of 261 days (range 37–997 days).
Six patients required a total of 16 subsequent procedures after initial recovery from CRS/HIPEC: 4 procedures for recurrence and 12 for other indications. The median time to any subsequent procedure was 9 months (range, 1–17 months). The most common benign indication for surgical intervention after CRS/HIPEC was adhesive bowel obstruction (N=3). Two of the three patients operated on for bowel obstruction were found to have sclerosing peritonitis with fused abdominal cavities consisting of dense, fibrotic adhesions requiring several hours of sharp adhesiolysis to relieve the obstruction. Two patients developed clinically significant hemorrhagic cystitis, one of whom developed a bladder rupture and required cystectomy and permanent bilateral nephrostomy tube placement. Two of the 4 patients with diverting ileostomies underwent reversal, one of which developed an anastomotic leak and required re-exploration. The remaining two developed recurrent disease and ostomy reversal was deferred secondary to additional chemotherapy (Table 3).
Table 3:
Surgical management of long-term morbidity after CRS/HIPEC for DSRCT. Variable N (%)
| Variable | N (%) |
|---|---|
| Complication Description | |
| Bowel Obstruction | 3 |
| Hemorrhagic Cystitis | 2 |
| Sclerosing Peritonitis | 2 |
| Gastroparesis | 1 |
| Subsequent Procedures | 6 (67) |
| Total Number of Subsequent Procedures | 16 |
| Procedure for Recurrence | 4 |
| Bowel Resection/Diaphragm Resection/ HIPEC | 1 |
| Liver Resection | 1 |
| Diaphragm Resection | 1 |
| Splenectomy/Lymphadenectomy | 1 |
| Procedures for Benign Indication(s) | 12 |
| Adhesiolysis | 3 |
| Stoma Reversal | 2 |
| Bladder Packing/Pelvic Packing* | 2 |
| Gastrostomy Tube | 2 |
| Cystectomy* | 1 |
| Right Colectomy** | 1 |
| Wound Debridement | 1 |
| Additional Hospitalizations | 8 (89) |
| Number of Hospitalizations/Patient | 8 (5–23) |
| Total Length of Additional Hospitalizations (days) | 76 (32–298) |
| Length of Total Parenteral Nutrition (days) | 261 (37–997) |
Results presented as Frequency (Percentage) or Median (Range)
-Bladder packing and subtotal cystectomy was performed for complications related to hemorrhagic cystitis.
-R colectomy was performed for an anastomotic leak after loop ileostomy reversal.
DISCUSSION
DSRCT is an extremely aggressive malignancy with poor median survival, ranging from 17–25 months.[19, 20] A previous report of 11 DSRCT patients from our institution demonstrated that long-term survival is possible, with 2 patients surviving more than 7 years from diagnosis, but those who have an incomplete response to initial chemotherapy and surgery have a dismal outcome.[21] More recently, aggressive multimodality therapy consisting of multi-agent neoadjuvant chemotherapy, complete cytoreduction with HIPEC, adjuvant WART and chemotherapy has become a potentially acceptable treatment approach.[5, 11, 14, 22, 23] Using this treatment approach, we identified a median OS of 45 months and 3-year OS of 55%. In a retrospective French nationwide study of multiple sarcoma databases, Honore et. al. observed a median survival of 42 months with a 54% 2-year survival for patients with DSRCT treated with multimodal therapy.[14] Hayes-Jordan et. al. reported a recent Phase 2 trial for sarcoma patients treated with CRS/HIPEC and demonstrated a median OS of 58 months and 3-year OS of 79% for DSRCT.[11]
While the oncologic outcomes from our institution are comparable to other established centers, we did not anticipate the degree of short- and long-term morbidity as a result of this ambitious treatment approach. CRS/HIPEC is an inherently aggressive procedure associated with long operative times, significant blood loss and potential morbidity. The possibility of morbidity is cumulative when considering the duration of neoadjuvant multi-agent chemotherapy administered before surgery (6.5 months in this series) as well as the adjuvant chemotherapy and WART administered after recovery from CRS/HIPEC. The most profound short-term complication experienced was seemingly related to cisplatin use during HIPEC, resulting in acute kidney injury (AKI) in two patients. Cisplatin is nephrotoxic and requires an aggressive pre-operative and post-operative fluid management strategy utilizing sodium thiosulfate to mitigate the effect of AKI.[24] Further development of more effective, less toxic HIPEC agents is warranted.
Long-term complications requiring additional treatment were also clinically significant in this series. Not previously described, poor oral intake was experienced by many of the patients and most needed prolonged parenteral nutrition. The most impactful long-term complications experienced included gastroparesis, adhesive bowel obstruction secondary to sclerosing peritonitis, and hemorrhagic cystitis. Each of these late treatment-related complications occurred a year or more after CRS/HIPEC, after completion of all treatment modalities, and required multiple additional hospitalizations and procedures. Previously, Osborne et al. noted that 13 % of patients developed late genitourinary and gastrointestinal toxicity after sequential multimodality treatment.[5] In recent years patients may have not survived long enough to experience many of the late effects of such an aggressive treatment approach. These late effects highlight the need for investigation of a more effective, less toxic, multimodality treatment approach.
The completeness of cytoreduction score has been shown to be a reliable prognostic factor for outcome after CRS/HIPEC.[7, 11, 14, 23] Complete cytoreduction (CCR 0/1) was performed in 9 of the 10 CRS/HIPEC procedures in this series with all visible disease resected at the time of surgery. However, we did not demonstrate a difference in OS based on CCR score (Figure 2C), likely related to the small number of patients in each group. We did, however, observe an improved OS for those with a lower PCI (Figure 2D). In a previous review of the first 50 CRS/HIPEC for pediatric malignancies, a lower PCI was found to be associated with a disease-free survival benefit but without an impact on OS.[22] A more extensive review of 26 patients (27 HIPECs), however, demonstrated no predictive impact of PCI on RFS or OS.[23] Larger studies are needed to clarify the impact of PCI and CCR score on overall outcome for DSRCT.
Extra-abdominal disease is a common finding at initial presentation for DSRCT. Similar to the inclusion criteria for the phase II study of sarcoma patients, we do not perform CRS/HIPEC in the presence of PET-avid extra-abdominal disease.[11] A recently proposed staging system evaluating factors including the burden of disease (PCI), liver metastasis and extra-abdominal disease has been developed to stratify outcome based on extent of disease at presentation, with extra-abdominal disease being the defining criterion for stage IV disease.[10] Neither DSRCT stage nor the presence of extra-abdominal disease had a significant impact on OS in this series (Figure 2A and 2B). These results are in contrast to the larger MD Anderson series in which DSRCT stage had an impact on RFS and extra-abdominal disease had a significant impact on both RFS and OS.[23] A multi-institution pooled analysis would likely further clarify and strengthen the association between extra-abdominal disease and outcome, given the small numbers of patients analyzed in most institutional series.[14]
There are several limitations of this study. The results are non-randomized and represent a retrospective review from a single institution. Ultimately, the small sample size leads to an underpowered analysis. However, the trends observed in both short- and long-term morbidity coupled with the great distances patients travel for treatment in specialized centers underscores the need for further study of this disease. The distance travelled for patients treated in this series was similar to the distances travelled at high volume centers recently reported in a nationwide study of DSRCT.[25] Because of location and distances travelled to undergo CRS/HIPEC, some received neoadjuvant and/or adjuvant treatment at facilities closer to their home. The HIPEC agent was not standardized and may have affected patterns of intraperitoneal failure after CRS/HIPEC. While cisplatin is the preferred agent for DSRCT, given the patterns of recurrence and cumulative late-complications after multimodality treatment, further study of alternative agents is warranted. Lastly, because of the rare nature, an international registry is warranted to better characterize this disease and allow prospective multicenter clinical trials to improve outcome with less morbidity.
CONCLUSION
DSRCT is a rare aggressive sarcoma affecting primarily adolescent males and young adults. Multimodality therapy consisting of multi-agent neoadjuvant chemotherapy, CRS/HIPEC, adjuvant chemotherapy and radiation therapy is associated with potential cumulative toxicity and recurrence after resection is common. Prolonged parenteral nutrition may be necessary and late gastrointestinal and genitourinary complications may require additional treatment.
SYNOPSIS.
Multimodal therapy for desmoplastic small round cell tumor consisting of multi-agent neoadjuvant chemotherapy, cytoreduction, hyperthermic intraperitoneal chemotherapy, adjuvant chemotherapy and radiation therapy is associated with potential cumulative toxicity. Late-term gastrointestinal and genitourinary complications may require additional treatment.
ACKNOWLDEGEMENTS:
The authors wish to thank Lynn Wynn, Amy Kimble and Dina Darby for the care of all patients treated in this series.
FUNDING SOURCES: This work was supported in part by the R25CA23944 grant from the National Cancer Institute.
Footnotes
DISCLOSURES: All authors declare no conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Gerald WL, Rosai J. Case 2. Desmoplastic small cell tumor with divergent differentiation. Pediatr Pathol 1989; 9: 177–183. [DOI] [PubMed] [Google Scholar]
- 2.Lettieri CK, Garcia-Filion P, Hingorani P. Incidence and outcomes of desmoplastic small round cell tumor: results from the surveillance, epidemiology, and end results database. J Cancer Epidemiol 2014; 2014: 680126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerald WL, Miller HK, Battifora H et al. Intra-abdominal desmoplastic small round-cell tumor. Report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. Am J Surg Pathol 1991; 15: 499–513. [PubMed] [Google Scholar]
- 4.Schwarz RE, Gerald WL, Kushner BH et al. Desmoplastic small round cell tumors: prognostic indicators and results of surgical management. Ann Surg Oncol 1998; 5: 416–422. [DOI] [PubMed] [Google Scholar]
- 5.Osborne EM, Briere TM, Hayes-Jordan A et al. Survival and toxicity following sequential multimodality treatment including whole abdominopelvic radiotherapy for patients with desmoplastic small round cell tumor. Radiother Oncol 2016; 119: 40–44. [DOI] [PubMed] [Google Scholar]
- 6.Kushner BH, LaQuaglia MP, Wollner N et al. Desmoplastic small round-cell tumor: prolonged progression-free survival with aggressive multimodality therapy. J Clin Oncol 1996; 14: 1526–1531. [DOI] [PubMed] [Google Scholar]
- 7.Lal DR, Su WT, Wolden SL et al. Results of multimodal treatment for desmoplastic small round cell tumors. J Pediatr Surg 2005; 40: 251–255. [DOI] [PubMed] [Google Scholar]
- 8.Atallah V, Honore C, Orbach D et al. Role of Adjuvant Radiation Therapy After Surgery for Abdominal Desmoplastic Small Round Cell Tumors. Int J Radiat Oncol Biol Phys 2016; 95: 1244–1253. [DOI] [PubMed] [Google Scholar]
- 9.Pinnix CC, Fontanilla HP, Hayes-Jordan A et al. Whole abdominopelvic intensity-modulated radiation therapy for desmoplastic small round cell tumor after surgery. Int J Radiat Oncol Biol Phys 2012; 83: 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayes-Jordan A, Green H, Fitzgerald N et al. Novel treatment for desmoplastic small round cell tumor: hyperthermic intraperitoneal perfusion. J Pediatr Surg 2010; 45: 1000–1006. [DOI] [PubMed] [Google Scholar]
- 11.Hayes-Jordan AA, Coakley BA, Green HL et al. Desmoplastic Small Round Cell Tumor Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Results of a Phase 2 Trial. Ann Surg Oncol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scalabre A, Philippe-Chomette P, Passot G et al. Cytoreductive surgery and hyperthermic intraperitoneal perfusion with chemotherapy in children with peritoneal tumor spread: A French nationwide study over 14 years. Pediatr Blood Cancer 2018; 65. [DOI] [PubMed] [Google Scholar]
- 13.Zmora O, Hayes-Jordan A, Nissan A et al. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for disseminated intraabdominal malignancies in children-a single-institution experience. J Pediatr Surg 2017. [DOI] [PubMed] [Google Scholar]
- 14.Honore C, Atallah V, Mir O et al. Abdominal desmoplastic small round cell tumor without extraperitoneal metastases: Is there a benefit for HIPEC after macroscopically complete cytoreductive surgery? PLoS One 2017; 12: e0171639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Msika S, Gruden E, Sarnacki S et al. Cytoreductive surgery associated to hyperthermic intraperitoneal chemoperfusion for desmoplastic round small cell tumor with peritoneal carcinomatosis in young patients. J Pediatr Surg 2010; 45: 1617–1621. [DOI] [PubMed] [Google Scholar]
- 16.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996; 82: 359–374. [DOI] [PubMed] [Google Scholar]
- 17.Glehen O, Kwiatkowski F, Sugarbaker PH et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004; 22: 3284–3292. [DOI] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dufresne A, Cassier P, Couraud L et al. Desmoplastic small round cell tumor: current management and recent findings. Sarcoma 2012; 2012: 714986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honore C, Amroun K, Vilcot L et al. Abdominal desmoplastic small round cell tumor: multimodal treatment combining chemotherapy, surgery, and radiotherapy is the best option. Ann Surg Oncol 2015; 22: 1073–1079. [DOI] [PubMed] [Google Scholar]
- 21.Saab R, Khoury JD, Krasin M et al. Desmoplastic small round cell tumor in childhood: the St. Jude Children’s Research Hospital experience. Pediatr Blood Cancer 2007; 49: 274–279. [DOI] [PubMed] [Google Scholar]
- 22.Hayes-Jordan A, Green H, Lin H et al. Cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for children, adolescents, and young adults: the first 50 cases. Ann Surg Oncol 2015; 22: 1726–1732. [DOI] [PubMed] [Google Scholar]
- 23.Hayes-Jordan A, Green HL, Lin H et al. Complete cytoreduction and HIPEC improves survival in desmoplastic small round cell tumor. Ann Surg Oncol 2014; 21: 220–224. [DOI] [PubMed] [Google Scholar]
- 24.Green H, Lin H, Owusu-Agyemang K et al. Perioperative Renal Protective Treatment Avoids Renal Toxicity in Pediatric and Adult Patients Undergoig HIPEC with Cisplatin. Journal of Pediatric Oncology 2014; 2: 10–16. [Google Scholar]
- 25.Stile ZE, Dickson PV, Glazer ES et al. Desmoplastic small round cell tumor: A nationwide study of a rare sarcoma. J Surg Oncol 2018. [DOI] [PubMed] [Google Scholar]