Figure 7.
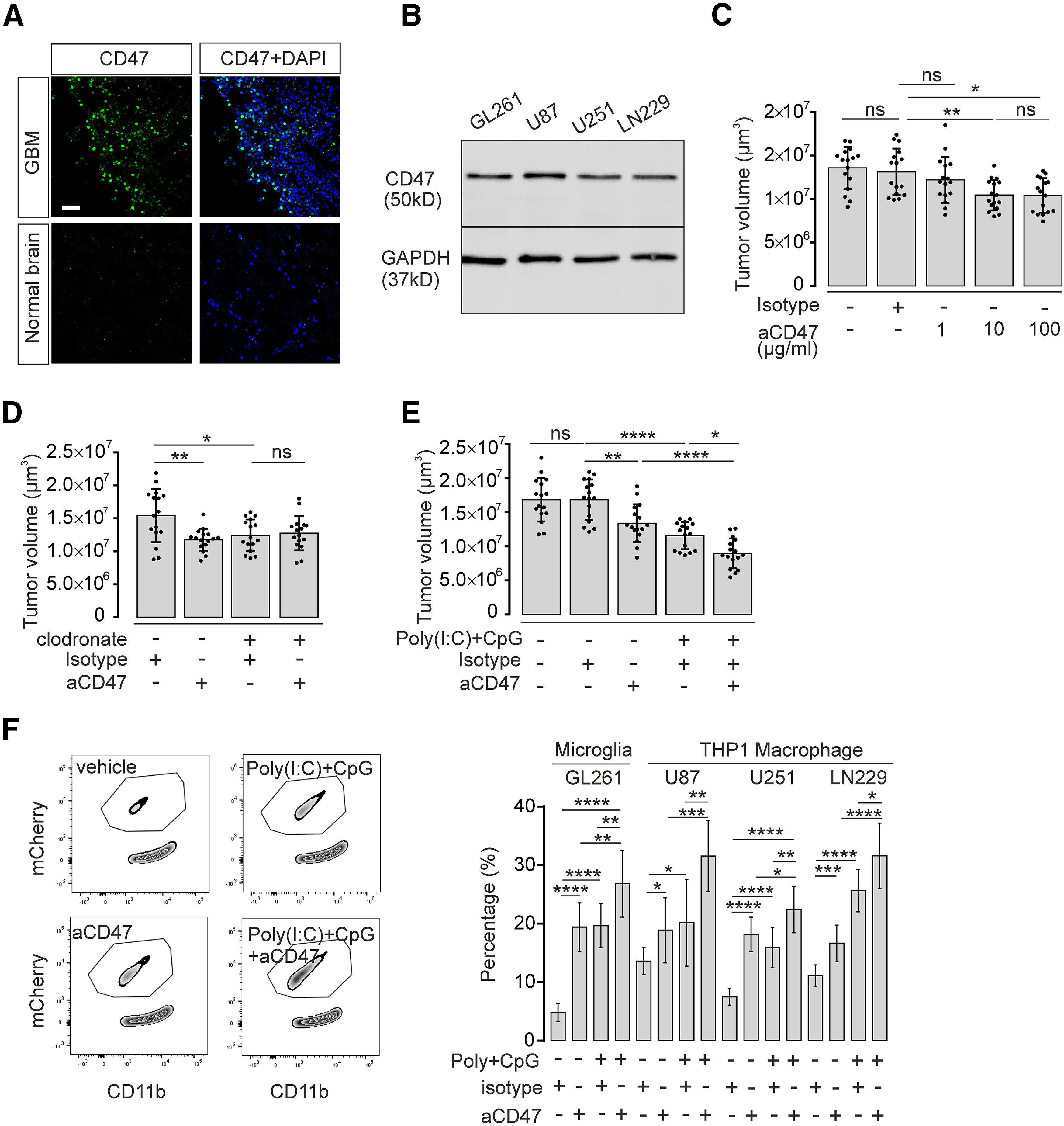
CD47 blockade and TLR3/TLR9 costimulation synergistically improve microglia/macrophage-induced tumor clearance. A, Immunofluorescence staining of CD47 expression (green) in human GBM tissue compared with normal human brain cortex tissue. Nuclei are counterstained with DAPI. Scale bar, 50 μm. B, CD47 protein level in murine GL261 and human U87, U251, and LN229 glioma cell lines was detected by Western blot. GAPDH expression served as reference. C, Organotypic brain slices inoculated with GL261mCherry cells were treated with 1, 10, or 100 µg/ml CD47 blocking antibody (aCD47) and compared with isotype control antibody. Tumor volumes were evaluated (n = 16 per group). D, Organotypic brain slices inoculated with GL261mCherry cells were pretreated with clodronate for 48 h to deplete intrinsic microglia. Subsequently, slices were treated with 10 µg/ml CD47 blocking antibody (aCD47) for 120 h and compared with isotype control antibody (n = 16 per group). E, Organotypic brain slices injected with GL261mCherry cells were treated with 10 µg/ml CD47 blocking antibody (aCD47) together with or without 10 µg/ml Poly(I:C) and 2 µM CpG, and tumor volumes were assessed (n = 16 per group). F, FACS assay was performed as described in Figure 4E to determine mCherry incorporation into cultured microglia treated with 10 µg/ml CD47 blocking antibody (aCD47), Poly(I:C) + CpG, or Poly(I:C) + CpG + CD47 blocking antibody (left). Right, Quantification of FACS data. Percentage of microglial cells that had incorporated mCherry material is given. In addition, using this paradigm described above, we tested the effect on the incorporation of the human glioma cell lines U87, U252, and LN229 into the macrophage cell line THP1. Isotype antibody served as a control (n = 9 per group). ns = no significance, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
