Abstract
Introduction:
The novel Coronavirus disease 2019 pandemic is sweeping through China, posing the greatest ever threat to its public health and economy. As a tertiary cancer center in Southwest China, we formulated and implemented an anti-infection protocol to prevent the spread of Coronavirus disease 2019 in our department.
Methods:
The anti-infection protocol divided patients into 3 categories, namely outpatients, inpatients, and patients receiving radiation therapy at our cancer center, and each category had a distinct anti-infection protocol to minimize the risk of Coronavirus disease 2019 transmission. In each category, the patients were classified into high-, intermediate-, and low-risk groups. Each risk group was managed differently. A survey of patient volume changes prior to and during the Coronavirus disease 2019 outbreak was performed.
Results:
We carried out the anti-infection protocol at our cancer center during the Coronavirus disease 2019 outbreak. We found that the total volume of both outpatient visits and inpatient treatment declined significantly depending on the conditions of each group. Radiation therapy and palliative service had the lowest and highest volume reductions at 58.3% and 100%, respectively. The decline in outpatient volumes was higher than the decline in inpatient treatment services (78.8% vs 71.8%). There was no Coronavirus disease 2019 cross-infection at our center, or Coronavirus disease 2019–related injury or death. The anti-infection protocol measures continue to be taken at the hospital even today but they have been modified depending on the prevalent local conditions.
Conclusions:
Challenges from the Coronavirus disease 2019 pandemic remain in our community. The anti-infection protocol implemented at our cancer center has been effective in preventing cross-infection. Whether our anti-infection protocol experience can be applied to curb the spread of the infection in other parts of the world remains to be tested.
Keywords: COVID-19, cancer center, chemotherapy, radiotherapy, NCP, AIP
Introduction
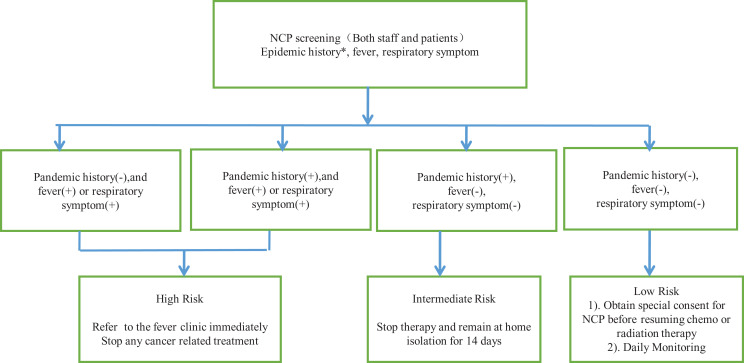
From December 2019 to February 2020, China faced the greatest ever threat to its population’s health and the country’s economy from the coronavirus disease 2019 (COVID-19) outbreak.1,2 The mechanism of transmission of the disease was gradually uncovered, which brought an immense challenge to medical staff in terms of how to prevent cross-infection. There is evidence that the novel COVID-19 spreads through human-to-human transmission, with the reproductive number (R0) for COVID-19, or the average number of people that an infected person can spread the virus to, estimated to range from 2.24 to 3.58, which indicates the potential for outbreaks.3,4 By February 8, there were reports of 34 598 diagnosed cases and 723 deaths from novel coronavirus pneumonia. The Chinese government took swift and stringent measures to contain the spread.5 As a cancer center in China, we faced challenges in keeping staff and patients safe, while continuing to provide our services. In this article, we outline the anti-infection protocol (AIP) implemented by the cancer center at Sichuan Provincial People’s Hospital to control the spread of COVID-19 at the center and discuss its implications for oncology departments in general (Figure 1).
Figure 1.
Schema of anti-infection protocol (AIP). *Pandemic history is defined as having had contact with an infected person/visited Wuhan, the epicenter of Coronavirus disease 2019 (COVID-19).
Methods
Our hospital is located in Chengdu, Sichuan Province, which has a population of 12.281 million. The annual outpatient visits to the oncology department are 34 173, and the annual outpatient visits to the hospital are 640 000. Our hospital has 4123 open beds and 106 beds for the oncology department. Following hospital procedure guidelines, we implemented an AIP that took into account the specific circumstances of our department. Then, we conducted a survey to compare outpatient visits and inpatient volumes prior to and during the COVID-19 pandemic to determine whether there was cross-infection and COVID-19-related injury or death at our cancer center at the time of this writing.
Anti-Infection Protocol for Cancer Center Outpatients
All patients entering the hospital clinic were required to undergo a temperature check and screening to see whether they had a history of visiting a pandemic affected area. Yellow tape was put up as barricades at all but one entrance and one exit to our hospital, while private access was provided to medical staff, who were also required to undergo the same screening procedure although they were physically separated from patients and their companions. Masks were mandatory for all hospital personnel, including receptionists in outpatient settings. To reduce contamination, scheduled office visits were either canceled or rescheduled. For people who were allowed to visit, the screening procedures were performed prior to the visit, which included checking whether patients and their companions had an epidemiological and cluster history, but no symptoms such as shortness of breath, diarrhea, cough, or fever (≥37.3 °C). To prevent cross-infection, medical staff were given protective gear, including gowns, glasses, gloves, head covers, and masks. The oncology outpatient clinic stopped its infusion service.
To reduce the frequency of office visits, doctors were allowed to prescribe anticancer and related adjuvant drugs that would last up to 3 times longer than normal. Meanwhile, medical staff also adopted flexible work hours to reduce their time of hospital stay.
Anti-Infection Protocol for Radiation Therapy Center
Patients who started radiation therapy prior to the pandemic could continue therapy if the following criteria were met: (1) The patient was willing to undergo radiotherapy; (2) the patient had no history of visiting a COVID-19 affected region, no fever (≥37.3 °C), and no respiratory symptoms; and (3) the doctor-in-charge had fully evaluated outpatient radiotherapy patients. For outpatients who wished to continue receiving radiotherapy, doctors were required to check their temperature daily, and inquire about their respiratory symptoms and epidemiological and cluster history, with patients without any abnormal findings allowed to continue the treatment. Radiation therapists were advised to protect themselves in accordance with the previously mentioned guidelines.
All patients were treated by appointment only. Patients and medical staff who failed the epidemiological and cluster history screening but had no symptoms such as shortness of breath, diarrhea, cough, or fever (≥ 37.3 °C) had to remain in home isolation for 14 days. Therapy for such patients was discontinued. Patients and medical staff who failed the epidemiological and cluster history screening, while also exhibiting symptoms such as shortness of breath, diarrhea, cough, or fever (≥37.3 °C) were required to visit the fever clinic immediately. For patients who refused to provide history and cooperate with the examination, doctors were required to stop radiotherapy and inform them of possible violations of and their liability under the Law on Prevention and Treatment of Infectious Diseases.
Anti-Infection Protocol for Cancer Center Inpatients
According to the unified arrangement protocol of the hospital, we consolidated and reduced the number of inpatient wards, suspended new admissions for conventional treatment including chemotherapy, immunotherapy, and radiotherapy, and halted the good clinical practice (GCP) study protocol treatment. The doctors in charge and outpatient doctors had the responsibility to tell the patients, barring those in a critical condition, and their families that their conventional treatment had to be postponed because of the COVID-19 pandemic, and most patients fully understood. All patients had to wear masks properly, while medical staff had to follow the protective measures as outlined earlier. The temperature of all inpatients was checked twice daily. To reduce the density of personnel in the ward, it was recommended that family members or caregivers not be allowed to accompany patients during hospitalization and radiotherapy, except for those of advanced age or those who needed special care. Patients had to take in-house meals, and no outpatient food was allowed in the wards.
Results
To date, there have been 561 confirmed COVID-19 cases and 3 deaths in Sichuan Province, including 166 cases and 3 deaths in Chengdu City. Since the implementation of the AIP, new hospital visits and follow-ups have declined dramatically. Tables 1 and 2 show the details of the volume changes (Tables 1 and 2).
Table 1.
Comparison of Outpatient Visits Prior to and During the COVID-19 Pandemic.
| Items | Prior to outbreak (number/week) | During outbreak (number/week) | Volume reduction (%) |
|---|---|---|---|
| New patients | 346 | 91 | 73.7 |
| Follow-up | 335 | 52 | 84.5 |
| Radiation | 180 | 65 | 63.9 |
| Outpatient infusion | 120 | 0 | 100 |
| Subtotal | 981 | 208 | 78.8 |
Abbreviation: COVID-19, Coronavirus disease 2019.
Table 2.
Comparison of Inpatient Care Prior to and During the COVID-19 Pandemic.
| Items | Prior to outbreak (number/week) | During outbreak (number/week) | Volume reduction (%) |
|---|---|---|---|
| Infusion for chemotherapy or immunotherapy | 116 | 31 | 73.3 |
| Anti-infection or pain control | 12 | 4 | 66.7 |
| Radiation | 12 | 5 | 58.3 |
| Palliative | 2 | 0 | 100 |
| Subtotal | 142 | 40 | 71.8 |
Abbreviation: COVID-19, Coronavirus disease 2019.
As a result of the AIP, the total volumes of both outpatient visits and inpatient treatment declined significantly, including a 100% reduction in palliative inpatient care and outpatient infusion. Overall, outpatient visits and inpatient services declined 78.8% and 71.8%, respectively. Because of the requirements for continuity of care, radiotherapy had a lower volume reduction than infusion for chemotherapy or immunotherapy in both outpatients (63.9% vs 100%) and inpatients (58.3% vs 73.3%). Among outpatients, new patient visits declined by 73.7% and follow-up visits by 84.5%. Among inpatients, the majority required either chemotherapy or immunotherapy, and their immune status was less sufficient compared with those receiving radiation therapy; thus, more patients chose to either delay the next cycle of treatment or cancel it (73.3%). Other patients, such as those receiving radiation, pain control, or other palliative care, accounted for only 18% (26 of 142). There was no cross-infection of COVID-19 or related injury or death.
As of May 2, 2020, the AIP measures were still being taken at the hospital, but they have been modified in line with the prevalent local conditions. We resumed chemotherapy, immunotherapy, GCP study protocol treatment, and new radiotherapy following a rigorous evaluation to rule out patients with COVID-19. The health care numbers have returned to 80% of the previous figures. During the study period, we performed nucleic acid testing for COVID-19 only in patients with epidemiological and cluster history, but no symptoms such as shortness of breath, diarrhea, cough, or fever (≥ 37.3 °C). However, we have now introduced nucleic acid testing for all inpatients. A total of 3 cases were confirmed at our hospital, but none in the oncology patient population. No COVID-19 positive case has been diagnosed at the hospital since February 11, 2020.
The limitations of the study are that it (1) lacked a comparison group to demonstrate the efficacy of the AIP and (2) was a single-center experience. The viral pandemic is a public health emergency, which makes it difficult and also unethical to conduct a comparison study as it could put participants at risk. However, the findings of this retrospective study suggest that an immediate and protective AIP could contain the spread of the viral pandemic in a medical facility. How the pandemic will evolve in 2020 remained uncertain at the time of this writing, and our experience at a tertiary cancer center is only a small facet of the complex anti-infection phenomenon. We hope that the pandemic comes to an end soon so that the oncology community can resume its routine practice.
Conclusion
In the face of the challenges posed by the COVID-19 pandemic, our hospital successfully implemented an anti-infection protocol, which was effective in preventing cross-infection. Due to increasing globalization, new viral infections would be a huge challenge for medical personnel. Whether our AIP experience can be replicated in other parts of the world to prevent the spread of viral pandemics remains to be tested.
Acknowledgments
The authors would like to thank Editage (www.editage.com) for English language editing. The authors would like to thank X luo, HS Bai, MX Zhou, YM Sun, ZC Zhang, MW Wu, YY Shen for proofreading. The authors would like to express our appreciation to the following people, Hansong, Bai, Xin Luo, Mingxiu Zhou, who kindly contributed to the article.
Abbreviations
- AIP
anti-infection protocol
- COVID-19
coronavirus disease 2019
- GCP
good clinical practice
- NCP
novel coronavirus pneumonia.
Authors’ Note: Our study did not require an ethical board approval because it did not contain human or animal trials.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Research Grant from Sichuan Academy of Medical Sciences and Provincial People’s Hospital (grant numbers:30305031017P); National Science and Technology Bureau China (grant numbers 3035031263), Sichuan Science and Technology Office (grant numbers 3050410336).
ORCID iD: Ming Zeng  https://orcid.org/0000-0002-4294-2077
https://orcid.org/0000-0002-4294-2077
References
- 1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Eng J Med. 2020;382(13):1199–1207. doi:10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phan T. Novel coronavirus: from discovery to clinical diagnostics. Infect Genet Evol. 2020;79:104211 doi:10.1016/j.meegid.2020.104211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao S, Lin Q, Ran J, et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019 nCov) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–217. doi:10.1016/j.ijid.2020.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol. 2020;94(7):5 doi:10.1128/JVI.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323(8):709–710. doi:10.1001/jama.2020.1097 [DOI] [PubMed] [Google Scholar]