Abstract
Metal and metalloid contamination of soil and sediment is a widespread problem both in urban and rural areas throughout the United States (U.S. EPA, 2014). Beneficial use of waste by-products as amendments to remediate metal-contaminated soils and sediments can provide major economic and environmental advantages on both a site-specific and national scale. These waste by-products can also reduce our need to mine virgin materials or produce synthetic materials for amendments. Waste by-products must not be hazardous or pose unacceptable risk to human health and the environment, and should be a suitable replacement for virgin and synthetic materials. This review serves to present the state of science on in situ remediation of metal-contaminated soil and sediment and the potential for beneficial usage of waste by-product materials. Not all unintended consequences can be fully understood or predicted prior to implementing a treatment option, however some realized, and potentially unrealized, benefits and unintended consequences are explored.
Keywords: Amendments, metals, remediation, waste by-products
1. Introduction
Metal and metalloid contamination of soil and sediment is a widespread problem throughout the United States, both in urban and rural areas.[1] Lead (Pb), chromium (Cr), arsenic (As), zinc (Zn), cadmium (Cd), copper (Cu), mercury (Hg), and nickel (Ni) are the most commonly found metals and metalloids at contaminated sites.[2] Other less common metals found at the contaminated sites include antimony (Sb), barium (Ba), beryllium (Be), manganese (Mn), selenium (Se), silver (Ag), thallium (Tl), and vanadium (V). Certain metals (e.g., iron [Fe] or magnesium [Mg]) are naturally present in soil and sediment, but rarely at toxic levels. Contamination primarily results from anthropogenic pathways including industrial operations, energy generation (e.g., coal combustion), wastes from hospital and medical facilities, mining, manufacturing, historical use of leaded gasoline, and the use of synthetic products (e.g., paints, pesticides, batteries). Several of the common metal contaminants, such as Cu, Ni, and Zn, are essential micronutrients for human, plant, animal, and/or microorganism health. Some are toxic at even low concentrations (e.g., Pb, Ag, As, Hg), while others (e.g., rubidium [Rb], cesium [Cs], strontium [Sr]) are often neither toxic, nor essential.[3] The average concentration range of commonly found metals at contaminated sites with their categories are shown in Table 1[4]
Table 1.
Average, and common concentration range of selected elements in soil, and their categories.
| Metal | Selected average for soils (mg/kg) | Common range for soils (ppm or mg/kg) | Category |
|---|---|---|---|
| Antimony (Sb) | 1 | <0.05–630 | Toxic, metalloid |
| Arsenic (As) | 5 | <0.06–1,110 | Toxic, metalloid |
| Barium (Ba) | 512 | 5–4,850 | Toxic |
| Beryllium (Be) | 1 | <0.1–22 | Toxic |
| Cadmium (Cd) | 0.2 | <0.01–77 | Toxic |
| Cobalt (Co) | 8 | <0.1–216 | Essential metal with known biological functions |
| Chromium (Cr) | 31 | <1–4,120 | Toxic |
| Copper (Cu) | 15 | <0.05–5,090 | Essential with known biological function |
| Cesium (Cs) | <5 | <5–97 | Non-essential, non-toxic with no known biological functions |
| Lead (Pb) | 18 | <0.5–12,400 | Toxic |
| Manganese (Mn) | 495 | <5–7,780 | Essential element with known biological function |
| Mercury (Hg) | 0.03 | <0.01–56 | Toxic |
| Molybdenum (Mo) | 1 | <0.05–76 | Essential with known biological function |
| Nickel (Ni) | 14 | <0.5–2,310 | Essential with known biological function |
| Selenium (Se) | 0.2 | <0.2–8 | Toxic metalloid |
| Zinc (Zn) | 59 | <1–11,700 | Essential with known biological function |
The variation in metals’ concentration range is due to the inherent variation in soil lithology and parent materials, and does not dictate its mobility or bioavailability in soils and sediments. However, it does help determine the native concentrations and the percent attributed to the source of contamination. Therefore, differentiating between the baseline (or background) and human-induced concentrations is necessary prior to selecting any treatment technology.
Once introduced into the environment, metals will remain intact and will not degrade like organic molecules. Mercury and Se are exceptions, as they can be transformed and volatilized by specific microorganisms.[5] Accumulation of these metals in soil and sediment may cause adverse effects on soil/sediment health, food quality, human health, and the ecological receptors through various pathways. This may include food chain (e.g., soil → plant → animal → human), drinking of contaminated groundwater and/or surface water, low food quality (e.g., safety and marketability), potential reduction in agricultural land causing food insecurity, and associated land use problems (e.g., vacancy, zoning restrictions).[6-8]
Traditionally, mitigation of metal-contaminated soils and sediments has been achieved through excavation and disposal in a landfill, and/or capping systems. Excavation requires replacement with clean soil, sourced from another location, making this an unsustainable remediation approach. Capping systems are used to provide a physical barrier to restrict access to contaminated media or to inhibit surface water infiltration to prevent the further release of contaminants to the surrounding surface or groundwater. Capping also controls gas and odor emissions, and reduces the risks associated with dermal contact/and or incidental ingestion of surface soils.[9] Alternatively, immobilization techniques are designed to reduce the mobility of contaminants by altering physical or leaching characteristics of the contaminated matrix. Mobility is usually decreased by physically restricting the contact between contaminant and the surrounding groundwater, or by chemically altering the contaminant to make it more stable with respect to dissolution in groundwater or ecoreceptor bioavailability. Potential immobilization mechanisms include: solidification/stabilization (S/S), chemical reduction, oxidation, phytostabilization, and biological stabilization. Most immobilization technologies can be performed ex situ or in situ. Ex situ treatment options remove and/or degrade the contaminant above ground. However, it usually takes a longer period of time, and often creates the burden of additional treatment or disposal of the contaminated material to an offsite location. In situ processes are considered a more environmentally friendly and less intrusive treatment method compared to traditional remediation techniques, like excavation and capping,[9-12] and although their implementations are site specific, they are often preferred due to lower labor and energy requirements. In situ immobilization of metals in soil and sediment has been practiced via addition of organic, synthetic, or mineral-based amendments, which can complement natural attenuation processes (i.e., sorption, precipitation, and complexation). The remediation of metal-contaminated soil and sediment through in situ amendment application can be challenging and costly because of a variety of factors that influence the specific immobilization mechanisms and geochemical properties (pH, oxidation-reduction potential (ORP), mineralogy, conductivity), which come into play when amendments are applied in any specific treatment.
When considering the application of amendments generated from waste by-products, a thorough understanding of the physical and chemical properties is required. Depending on the physical and chemical properties of the amendment and the receiving environment, one or more immobilizing mechanisms may be responsible for a specific metal. In general, the predominant mechanism by which metals are immobilized through the addition of either inorganic (e.g., fly ash, slag, zeolites); organic (e.g., biosolids, manures, paper pulp); or a combination of both inorganic and organic by-product amendment types is through the precipitation of hydroxides.[13] Unintended consequences, whether beneficial or adverse, may result from the application of an amendment and must be considered when deciding on an amendment to use at a specific site. An example of an unintended adverse consequence is the addition of phosphate amendments to Pb-contaminated soil, which has been shown to decrease the mobility and the bioavailability of Pb; however, phosphate additions to Pb-contaminated soil that also contain elevated concentrations of antimony (Sb) and arsenic (As) can greatly increase the mobility of Sb and As.[14] Arsenic and phosphate are known to compete for reaction sites, and Sb is similar to As in chemical behavior. Numerous by-product amendments that can be categorized as waste by-products (from industrial or other operations) have been used for metal immobilization, including organic products (e.g., biosolids, manures, paper mill sludges); liming/alkaline products (e.g., cement kiln dust [CKD], coal combustion residuals [CCRs], red mud); and mineral/inorganic products (e.g., foundry sand, gypsum, steel slag). Waste by-products are often valuable resources, but are typically disposed of in a landfill in the lack of a better or more cost-effective usage. Beneficial use of waste by-products as amendments for metal immobilization in contaminated soil and sediment has several advantages as it provides the potential to replace virgin and synthetically produced amendments, recycle what would otherwise be disposed, and potentially create or strengthen the existing economic markets. The objectives of this review article are to: (1) summarize the current state of the science on in situ treatment of metal-contaminated soils and sediments; (2) review the more recent use of non-municipal and non-hazardous waste by-products for use as soil and sediment amendments; and (3) identify physical and chemical properties that are indicative of the success or effectiveness of using a specific amendment to treat metals in contaminated soils or sediments.
2. Overview of treatment technologies
Treatment methods for soil are generally similar to those of sediment, although fewer remediation technologies for sediment are both commercially available and cost-effective.[11,12,15] A summary of remedial options and technologies specifically used for metal-contaminated soil and sediment is presented in Fig. 1.
Figure 1.
Remedial options for metal-contaminated soil and sediment.
The remedial options presented in Fig. 1 can be applied in combination or separately, as well as in situ or ex situ, depending on the nature and the extent of contamination and remedial goals. In situ, or in its current location, indicates that the contaminated soil is treated in its original place; neither excavated nor moved, and remains in the subsurface. In situ treatment is often used to reduce metal leaching and metal toxicity/bioavailability, and establish vegetation at the contaminated site to reduce wind and water movement (as applicable) of metal-laden particles. Ex situ, or offsite, means that the contaminated soil is moved or excavated from the original site or subsurface for treatment and disposal, or for replacement. Nearly all ex situ technologies tend to be costly compared to in situ technologies due to the required operation and maintenance costs, aboveground technology needs, and labor. Ex situ treatment may take longer and often create the burden of additional treatment or disposal of the contaminated material or generated waste to an offsite location.[16]
3. Factors impacting the treated metals, and mechanisms involved in immobilization processes
In addition to the challenges associated with onsite/offsite treatment technologies, it is critical to understand the physical and chemical properties of soils/sediments at a particular contaminated site prior to application of any amendment. Soil structure, texture, surface area, bulk density, and composition are important physical properties that may further impact soil/sediment chemical properties. The fate and transport of metals highly depend upon the chemical and mineralogical properties such as particle size distribution, pH, salinity, nutrients, temperature, cation exchange capacity (CEC), soil moisture contents, organic carbon (OC) and organic matter (OM) content, reactivity of metals, concentration of organic and inorganic ligands, competing ions, colloid formation, and redox reactions.[17,18] The first major step in identifying amendments, and specifically candidate waste by-products to use as an amendment for in situ remediation of metal-contaminated soils and sediments, is to characterize its physical, chemical, and mineralogical properties. This review presents physical, chemical, and mineralogical properties of a variety of amendments included in peer-reviewed literatures and government (US EPA) publications to better understand the success factors of specific amendments and identify similarities across amendment types.
In general, the toxicity, mobility, and reactivity of metals depend on the metal’s distribution and its speciation. In soils and sediments, metals exist in various physiochemical forms such as dissolved, colloidal, exchangeable, adsorbed, organic complexes, precipitates, and as incorporated in the structure of secondary minerals.[19,20] Metal ions can be retained in soils and sediments largely by (ad)sorption, precipitation, complexation, and chelation reactions, thereby making them unavailable (to varying degrees) for human and plant uptake, as well as leaching to the groundwater. More details on common factors affecting the mobilization of metals in soils and sediments are provided in Table S1.
3.1. Sorption
Sorption interactions generally operate among all phases present in any subsurface system and is defined as the accumulation of matter at the interface between the aqueous solution phase and a solid sorbent phase.[21] Solutes that undergo sorption are termed as sorbates, sorbing phase is the sorbent, and the primary phase from which sorption occurs is the solution or solvent. Absorption and adsorption are two broad categories of the sorption phenomenon. Absorption is a process in which a solute is transferred from one phase to another that interpenetrates the sorbent phase by at least several nanometers. Adsorption is a surface-based process and refers to the adhesion of atoms, ions, or molecules from a gas, liquid, or dissolved solid onto a surface. Such accumulation is generally restricted to a surface or interface between the solution and adsorbent. Adsorption is the predominant mechanism by which metals are immobilized through in situ remediation. Three loosely defined categories of adsorption—physical, chemical, and exchange (electrostatic) adsorption—are differentiated according to the class of attractive force that predominates.[21] Physical adsorption is associated with van der Waals attraction between adsorbate and adsorbent. The attraction is not fixed to a specific site and the adsorbate is relatively free to move on the surface. This is relatively weak, reversible, and capable of multilayer adsorption. Chemical adsorption involves chemical bonding between the metal ions and the sorption sites on soil particle surfaces, and the adsorbed atoms/molecules are bound to specific chemically reactive sites on the surface and are not free to move. There is a high degree of specificity and typically a monolayer is formed, therefore chemical adsorption is seldom fully reversible. Exchange adsorption (ion exchange) is associated with electrostatic interactions due to charged sites on the surface.[16]
3.2. Precipitation
Precipitation is classified as a separate process; however, it is preceded by sorption reactions and occurs with an additional variation of the sorption process. Precipitation reactions occur when the solution becomes supersaturated with respect to the solid phase of the specific element of interest through either a homo-or hetero-aggregation process. Homo-aggregation precipitation occurs via nucleation of the supersaturated phase within the soil solution, while hetero-aggregation refers to the nucleation of a precipitate at the surface of another material where an element first adsorbs onto the surface of a soil particle followed by nucleation of the phase. Trace elements such as Zn, Ni, Cr, and Pb can precipitate onto a soil particle under specific reaction conditions and in the presence of reactive soil particle surfaces. A second form of hetero-aggregation involves co-precipitation, which involves the incorporation of a trace element into a mineral structure during solid-state solution formation and the recrystallization of minerals (e.g., the incorporation of Sr or Zn into a carbonate precipitate). Precipitation as metal phosphates is considered to be one of the major mechanisms for the P-induced immobilization of metals.[22]
In a bench-scale study, Jardine et al.[14] (Fig. 2) found that the addition of soluble Fe(II) and Fe(III) salts to soil containing Fe and Al oxides were more effective than metallic Fe in reducing As bioaccessibility. Adding soluble Fe(III) salts to contaminated soil caused a decrease in soil As bioaccessibility by increasing the Fe(III) (hydr)oxide content via precipitation reactions. The freshly precipitated amorphous Fe oxides provide significant surface area and charge to strongly bind As(III) and As(V), thus making it less bioavailable in soil. When soil moisture was ≥30%, the addition of Fe(III) amendments indicated that the reaction can occur in situ.[14] Similarly, Cr(III), which is highly reactive in soil systems, readily precipitates with soil Fe oxides and suggests that the transformation of Cr(VI) to Cr(III) coupled with the surface reactivity of Cr(III) significantly decreases Cr(VI) bioaccessibility.[14] Addition of organic amendments as an electron donor influences the soil microorganisms involved in the reduction of chromate Cr(VI) to chromite Cr(III), thereby facilitating its adsorption/precipitation reactions.[23]
Figure 2.
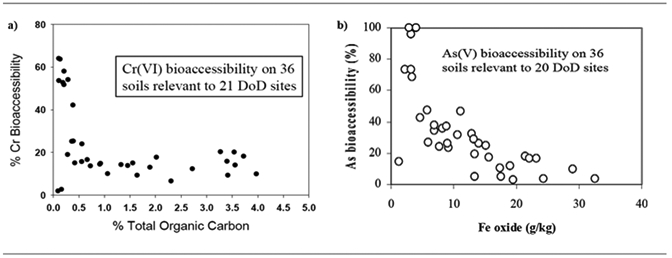
Effects of bioaccessibility of Cr and As from OM additions to Cr-contaminated soil (a) and Fe oxides to As-contaminated soil (b).[14]
3.3. Complexation and chelation
A complex consists of a central metal atom to which neutral or negatively charged ligands possessing electron donor properties are bonded. The resulting complex may be neutral, positive, or negative. Complexation reactions, in the context of metal remediation, are represented as Metal (acid) + Ligand (base) = Metal Complex. The general order of affinity for complexation of metal cations with OM is as follows: Cu2+ > Hg2+ > Cd2+ > Fe2+ > Pb2+ > Ni2+ > Co2+ > Mn2+ > Zn2+.[24] While most trace metals have a high affinity for complexation with OM, the formation of the complex is controlled by solution pH, ionic strength, redox potential, dominant cation, nature of the metal species, soil surface properties, and type and amount of inorganic and organic ligands present in the soil solution.
A special case of complexation that forms strong complexes when a ligand is bound to a metal ion in two or more places is called chelation. Stability increases with the number of chelating sites available on the ligand. The organic component of soil constituents has a high affinity for metal cations due to the presence of ligands or functional groups that can form chelates with metals.[25] With increasing pH, the carboxyl, phenolic, alcoholic, and carbonyl functional groups in soil OM dissociate, thereby increasing the affinity of the ligand for metal cations. Metals that form stable complexes with soluble OM are likely to be mobile in soil and sediment.[26] Attenuation of a metal complex may be enhanced when the complexing ligand is adsorbed onto a mineral or organic surface as the adsorbed ligand can serve as a site for metal retention.[26,27] Complexation may have strong influence on a metal’s distribution, fate/transport, and biochemical effects including plant uptake, toxicity, and bioavailability.[18,28]
Thus, mechanisms and factors involved in immobilization of metals in soils and sediments will provide significant information for amendment applications (Table S2).
4. Soil and sediment amendments
The occurrence of co-contaminants is always challenging and may require a more protective solution to counter unintended adverse consequences.[11] For example, the co-occurrence of Pb–As is common. In situ stabilization of soil Pb using P amendments, such as phosphate fertilizers and phosphate rock, have been suggested as a cost-effective and less-disruptive alternative for remediating Pb in soil relative to several other commonly used methods.[30] While the addition of P compounds may immobilize Pb, it has been found to increase the mobility of Sb and As because As and phosphate are known to compete for reaction sites, and Sb is similar to As in chemical behavior.[14,31,32] Therefore, common sense must prevail to utilize an amendment that is capable to sequester both Pb and As, such as iron-(oxy)hydroxide-based wastes, to reduce or eliminate the known unintended consequences. The three major categories have been used in the current review to categorize different types of amendments with specific physicochemical characteristics that are capable of immobilizing metals in soil and sediment: natural, synthetic, and waste by-product (further subcategorized into existing and potential). The characteristics of specific materials within each of these three categories may be similar and potentially interchangeable. Choosing a waste by-product amendment over other amendment materials (natural or synthetic) may be related to the availability of large volumes of the by-product (e.g., biosolids or food waste), economic costs or savings, proximity of the source to the remediation site, and/or a reduction in the use of virgin materials. As is the case for natural and synthetic amendments, the potential unintended consequences of a waste by-product (further reduced ecosystem service or increased human health risk) must be carefully considered.
4.1. Natural materials as amendments
Natural materials used as soil/sediment amendments include beneficial natural rock and earth materials recovered through mining activities. These materials include limestone, gypsum, phosphorite (phosphate rock), zeolite, apatite, bentonite, and other clays. Depending on the soil and sediment conditions, variation in particle size may be employed to increase the rate of chemical immobilization. Powdered and granulated materials are applied most often since grades (or particle sizes) typically available for these materials provide more reactive surfaces (higher surface area) to the amended soil. These natural materials have a broad range of applications for stabilizing different metal species in a broad range of soil conditions.
4.2. Synthetic materials as amendments
Synthetic materials are chemically engineered substances designed specifically for compatibility with soil/sediment conditions and the metal species present. Commercially developed synthetic materials include zero-valent iron, zeolites, and phosphates. Synthetic zeolites can be made from various materials but are often derived from fly ash.[34] Approximately 150 synthetic amendments are commercially available compared to 50 naturally occurring materials. Examples of commercially available synthetic phosphate-based amendments include EcoBond,[35] Fesi-Bond, and LockUpLead. Metal phosphate compounds that form with these types of products can exhibit low solubility. Unlike natural phosphate materials, or phosphate-containing wastes, the synthetic product bonds directly to the metal and may be less susceptible to pH-related deterioration that eventually enhances metals’ mobility.
Synthesized nanoparticles are another emerging type of amendment material. Researchers have successfully applied synthesized apatite nanoparticles to Pb-contaminated soil,[36] synthesized Fe phosphate (vivianite) nanoparticles to Cu(II)-contaminated soil,[37] and Fe-Mn binary oxide nanoparticles to As(III)-contaminated soil[38] in laboratory-based experiments. Nanomaterial amendments may not be cost effective as their production carry significant costs. To our knowledge, no study has examined the potential of metal-laden nanoparticle transport in soils. More details on the subcategories of natural and synthetic materials, including clay minerals, carbonates, sulfates, organoclay, phosphates, zeolites, iron-based minerals are provided in Table 2 Information on the wise use of amendments by considering the target contaminants, immobilization mechanisms involved, and beneficial uses to mitigate the potential unintended consequences are also presented.
Table 2.
Natural and synthetic materials used as soil and sediment amendments.
| Amendment category |
Amendment type | Source | Metal uptake mechanism |
Benefits of use | Limitations and unintended consequences |
Target contaminants |
|---|---|---|---|---|---|---|
| Natural | Clay minerals (e.g., palygorskite, Camontmorillonite bentonite) | Layered aluminosilicates: montmorillonite (bentonite deposits), vermiculite | Adsorption/ion exchange; possible surface precipitation | Natural expandable clays; used in geosynthetic sorptive mats | None identified | Cationic and anionic metal species |
| Natural and synthetic | Silica | Soluble alkali silicate salts: sodium metasilicate [Na2Si)3·H2O] | Adsorption, encapsulation | Forms amorphous silica or silica gel on aging; also used for permeability reduction | None identified | Cadmium (Cd), zinc (Zn), copper (Cu), and lead (Pb) |
| Natural | Carbonates | Calcite [CaCO3], dolomite [CaMg (CO3)2]; magnesite [MgCO3]; siderite [FeCO3]; soda ash [Na2CO3·H2O] | Solid solution carbonate mineral formation based on elevated pH | Used for acid neutralization and pH buffering | Some carbonate materials may contain concentrations of metals | Manganese (Mn), strontium (Sr), cesium (Cs), barium (Ba), aluminum (Al), cobalt (Co), nickel (Ni), Cu, Zn, thallium (Tl), Pb, bismuth (Bi), lanthanum (La), and uranium (U) |
| Natural | Sulfates | Gypsum, ferrous sulfate, and aluminum sulfate | Solid solution precipitation | Potential formation of ettringite-type phases; also used for permeability reduction | None identified | Pb, Cu, Cd, Co, Ni, Zn, and Mn |
| Natural | Lime | • Burnt lime (calcium oxide [CaO]) | Adsorption and solid solution precipitation | Highly soluble; produces alkaline pH and variable reaction products | Liming materials may contain metals; pH impact varies with source | Arsenic (As), Cd, chromium (Cr), Cu, Pb, Ni, and Zn |
| • Limestone (ground calcium carbonate [CaCO3]) | ||||||
| • Calcium hydroxide (Ca(OH)2) | ||||||
| Natural | Lime | Hydrated lime | Adsorption and solid solution precipitation | Highly soluble; produces alkaline pH and variable reaction products | Liming materials may contain metals; pH impact varies with source | As, Cd, Cr, Cu, Pb, Ni, and Zn |
| Natural | Portland-type cements | Limestone (calcium carbonate) mixed with small quantities of other materials, such as high-temperature calcium silicates (Ca3SiO5 and Ca2SiO4), calcium aluminate (Ca3Al2O6), and calcium aluminoferrite (Ca2AlFeO5) with magnesium (Mg), sodium (Na), and potassium (K) substitution | Solid solution, encapsulation, and precipitation | Used for in situ stabilization; highly reactive with water; products are mixtures of hydrated CaO–Al2O3–SiO2 phases; studied as an amendment for immobilization of problematic oxyanion contaminants such as arsenate | Some metals, such as arsenic(III), chromium(VI), and mercury, are not suitable for cement- and pozzolan-based treatments because they do not form highly insoluble hydroxides10,39 but with pretreatment or additives such as lime, this disadvantage can be overcome | Most metals, including Cd, Pb, Co, chromate, and molybdenum (Mo)40,41 |
| Natural and synthetic | Zeolites | Framework aluminosilicates: natural (clinoptilolite) and synthesized from coal fly ash | Adsorption/ion exchange | High surface area ion exchange; also mixed with cement | Efficacy is uncertain and is most successful when metal concentrations are low42 | Cu, Pb, Zn, Cd, and Ni |
| Natural (but commercially produced) | Slovakite (mixture of dolomite, bentonite, diatomic clays, alginate, and zeolite and clinker) | Manufactured by IPRES INziniering, Ltd. Bratislava; costs are about 700 Euros per ton for a granulation size of 0.2–0.5 mm | Adsorption | • Commercially available | May increase pH and lead to the remobilization of other constituents | Pb, iron (Fe), Cu, Al, Mn, and Zn44,45 |
| • May perform as well or better than apatite in reducing the mobility of Pb | ||||||
| • Shown to reduce the availability of Pb, Zn, Cu, and Cd in a lab environment43 | ||||||
| Natural | Phosphates | Solids: natural and synthetic apatite and other minerals; phosphate rock; chloropyromorphite has the lowest solubility of phosphorous (P) minerals and is most stable under favorable environmental conditions 46 | Precipitation, solid solution | • Solid sources include natural rock, bone meal, and fertilizers; most widely studied and used for Pb | Potential environmental and regulatory concerns over increased loadings of phosphate | Cd, Cu, Ni, Pb, and Zn |
| Synthetic | Phosphates | Soluble phosphate: phosphoric acid; and sodium, potassium, ammonium phosphate | Precipitation and solid solution47 | • Among the most widely studied and used for Pb; intended to sequester toxic divalent cations such as Pb2+ by precipitation of pure or substituted phases of the apatite group | ||
| Synthetic | Organoclay | Modified phyllosilicate derived from naturally occurring clays; cations in the interlayer of the mineral structure are exchanged for alkylammonium ions to create a surface with covalently linked organic molecules | Adsorption | • May be advantageous for soil containing mixed waste | Less useful in sediment because they are vulnerable to gas formation and may cause problems in areas of high groundwater flow12 | Pb, Hg48,49 |
| • The chelating ligand in the structure is the primary mechanism in which metals can be immobilized | Cu, Ni, Cd, Zn, Fe, Pb in sludge and wastewater50 | |||||
| • Also effective in removing oils, greases, and other high molecular weight/low solubility organics | ||||||
| Synthetic | Iron-based | Zero-valent iron (Fe0), Iron(III) oxides | Combination of precipitation and adsorption51 solid solution | Potential for oxidation state changes; surface-reactive amendment | • The application of ferrous sulfate has been found to free H2SO4 when it reacted to form Fe oxides, resulting in the mobilization of Mn in the trial soils52 | Chromate, As, mercury (Hg), Cu, Cd, Pb, Cr, U, Ni, Mn, and Al |
| The sorption behavior of Fe is related to pH; each ion has its own optimum pH range for metal adsorption34 | • Because each Fe ion has its own optimum pH range for metal adsorption,34 there may be unintended interactions if multiple metals are present |
5. Waste by-products as amendments
A single low-cost industrial by-product that possesses all of the physicochemical characteristics necessary for a range of applications does not exist. However, combinations of two or more by-products with natural and/or synthetic amendments are routinely used and can provide the necessary properties to ameliorate the challenges posed by metal contamination in soils and sediments. Waste by-products are viable alternatives to natural or synthetic materials under multiple scenarios. Some common examples of waste by-products used for immobilizing metals in soil include biosolids, gypsum, calcium compounds, yard wastes, and agricultural animal and plant wastes. Using waste by-products for in situ remediation of contaminated land is a beneficial use of the material that is likely to have both environmental and economic benefits. The economic advantage arise from the fact that waste by-products may be used at a low or no cost compared to commercially available materials, and the transportation cost can be minimized if waste by-products are obtained close to the location where the material will be applied.[11] The major environmental advantages associated with using waste by-products as amendments is that wastes are averted from being disposed of in landfills or surface impoundments and the need to mine or synthetically produce a similar material is reduced. The elimination of extraction of new raw material is a major factor in life cycle considerations from materials management perspective.
Waste by-products are categorized as organic, liming/alkaline, and mineral/inorganic, and can be further classified as either existing or emerging to be consistent with the U.S. EPA's 2007 report entitled The Use of Soil Amendments for Remediation, Revitalization, and Reuse.[11] A high-level summary on the waste by-product amendments identified is presented in Table S3.
5.1. Organic waste by-product amendments
In general, the sources of organic waste by-products are associated with sanitary waste (biosolids); yard wastes, left over materials from wood processing, plant residues from paper mills, agricultural wastes either as (i.e., manures), or composts made from one or more of these materials. When added to the soil, these materials generally provide OM and metal (oxy)hydroxides to the soil, promote enzymatic activity, and supply essential nutrients to the soil matrix. They also cycle nutrients (e.g., plant-available ammonia) and are important for nutrient mineralization of phosphates and sulfates. Enzymatic activities also play a role in ion exchange processes in the soil, especially in association with clays and soil colloids, which are important for metal fixation. Increased available nutrients afforded by organic amendments also promote the growth of soil microbial communities important for viable and sustainable plant cover. Soil microbe degradation by-products result in essential plant nutrients such as available C, N, O, P, K, and trace elements. Some organic waste by-product amendments contain significant C that is effective for adsorbing or chemically bonding many metal species.
5.1.1. Biosolids
Biosolids (BS) are commonly used in agricultural land application, land and mine site reclamation, and horticulture. They provide additional OM, N, P, and Fe, and also play a role in metal immobilization via sorption and complexation of the metal fractions associated with OM, metal oxides, or carbonates.[53] Mixed applications of biosolids and other waste by-products and non-waste by-products amendments (e.g., agricultural lime [ag lime], lime kiln dust [LKD], sugar beet lime [SBL], paper mill residues, and natural and iron-activated zeolite) are commonly used to immobilize Cd, Pb, Cu, As, and/or Zn in contaminated acid soil.[46,54-59] For example, Brown et al.[55] conducted a field study with mixed applications of biosolids combined with other waste by-product amendments (LKD alone, fine textured lime + biosolids/ag lime + biosolids/coarse textured lime + biosolids/SBL + BS, and LKD + BS) on fluvial mine tailings deposits to increase plant growth. Plant growth was greatest in the LKD + BS treatment with 92 ± 8% cover (Fig. 3). The application of LKD alone (43 ± 21%) and SBL + BS (50 ± 10%) also increased plant growth compared to previous years, and growth in all other amended treatments was similar with < 25% cover (Fig. 3).
Figure 3.
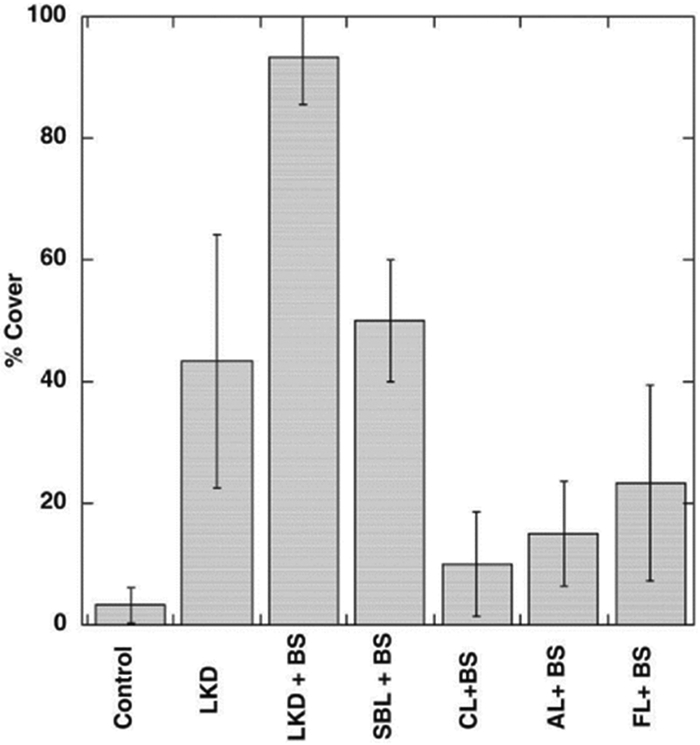
Percent cover for field plots on the alluvial tailings deposits. The measures were collected at the end of 2007 growing season, 7-year after plots were established. Means and standard deviation are shown (n = 3).[55] © 2009 American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America. Reproduced by permission of the American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America. Permission to reuse must be obtained from the rightsholder.
The success of a mixed biosolids application with respect to metal immobilization can vary greatly as would be expected when considering the variability in contaminated sites. Addition of biosolids or zeolites to seashell grit-amended soil did not exhibit further reduction in metal solubilization into soil pore water, but increased As solubilization due to excessive soil neutralization (pH > 6.5).[56]
5.1.2. Manures
Manure provide additional OM and nutrients to soils and are commonly used in agriculture and horticulture practices to improve soil structure, improve water-holding capacity, and enhance crop growth. Manure contains more soluble forms of N, which can lead to salt buildup and leaching losses. Only aged manures should be used for soil remedial purposes because fresh manures contain high levels of ammonia that may be phytotoxic to plants and soil microorganisms. Cow manure has been used to reduce the bioavailability of Zn and Mn by 91 and 95%, respectively, compared to non-amended control soils[53] due to the increase in soil pH, phosphate, and supplied essential plant nutrients.
5.1.3. Compost
Composts provide additional OM and nutrients to soil and are mainly used in agriculture and horticulture to improve soil structure, improve water-holding capacity, and enhance crop growth. In comparison with manures, compost contains less soluble forms of N. Biosolids compost used for soil remediation has been shown to reduce Cu bioavailability.[59] Vermicompost (composting enhancement through addition of worm populations) has been shown to have a high affinity and adsorption capacity for Cd.[60] The application of municipal solid waste (MSW) and biosolids compost to contaminated soil with a neutral pH was shown to induce an anaerobic environment, which, in turn, favored the conversion of As(V) to mobile As(III), which was then accumulated by a fern group (Pteridium aquilinum, Digitalis thapsi, and Cytisus scoparius).[34]
5.1.4. Yard and/or wood waste
Yard and wood waste from households, waste from tree removal and landscaping companies, or wood processing facilities are typically collected, then ground or shredded, and made available for purchase. The composition of these wastes can vary greatly depending on the source type, method of processing, and storage time and methods. The variability in composition, specifically the proportion of rock, mineral, OM, and moisture content[62,63] may change over time depending on the conditions and duration of storage.[64] This necessitates a thorough understanding of their chemical composition and physical properties. [61] Field trials conducted by Venner et al.[61] support the application of woody wastes for site rehabilitation if applied under conditions to avoid excessive leachate. In situ mixing of yard and/or wood waste with mineral soil has been shown to reduce bulk density and improve water-holding capacity; the additional fertilizer can compensate for N immobilization by wastes with high C:N ratios.[61] Surface application of certain amendments, including biosolids mixed with wood ash, resulted in significant decreases in subsoil acidity as well as subsoil extractable metals and was sufficient to restore a plant cover to metal-contaminated areas.[55]
5.1.5. Pulp and paper mill manufacturing waste by-products
Pulp and paper mill wastes are composed primarily of OM and are commonly applied to mine site soil to enhance revegetation as a source of nutrients. Specific waste by-products from pulp and paper manufacturing that have been used or have the potential to be used for in situ treatment of metals in soil/sediment include bleached pulp mill, kraft mill, deinking wastes, bark and wood chips, lime mud, waste paper, slaker grits, green liquor dregs, fiber sludge, xylogen (included in wastewater from paper mills), and sawdust. Application of these manufacturing wastes have resulted in the sorption of Zn and the attenuation of Cr and Cu[54] due to their high organic and clay content. Similar results were observed after applying paper mill water treatment sludge to soil contaminated with Cu, Zn, Ni, Pb, and Cd. Six months of sludge application showed a decrease of 30–50% in heavy metal mobile fractions (reductions in metal concentration were: Cu, 35%; Zn, 42%; Ni, 30%; Pb, 51%; Cd, 38%).[65] Enhanced sorption sites may have been made available on the solid phase of the contaminated soil after the sludge application.[65]
5.2. Liming/alkaline waste by-product amendments
Waste by-products under this subcategory can be organic (e.g., wood ash) material, inorganic (e.g., fly ash) material, or any material that contains labile Ca and Mg. Soil pH is a key chemical characteristic for the immobilization of numerous metal species. Soil and sediment contaminated with metals are often acidic, thus soil pH is a key chemical characteristic for the immobilization of numerous metal species.[11] Liming amendments can be used to raise the pH of contaminated soil to favor the formation of hydroxide, oxide, carbonate, and phosphate minerals, thereby reducing metals’ mobility and toxicity. Many adsorption sites on soil components are pH-dependent (e.g., OM, carbonates, metal oxides and clay minerals), and, as the pH decreases, the number of sites for cation adsorption decreases.[19,66]
While applying liming agents, care must be taken not to introduce amendments with phytotoxic characteristics, particularly when using industrial waste by-products. Phytotoxicity can occur from high metal contents and can be increased in acidic soil conditions coupled with other nutrient deficiencies. Phytotoxicity can result in stunted plant growth or plant death. Additionally, the toxicity of some metals (e.g., Al) can be reduced by the addition of residuals with high concentrations of specific cations (e.g., Mg, Ca, and K) that are constituents of some alkaline amendments.
5.2.1. Coal combustion residue (CCR as a liming agent)
Coal combustion residue (CCR), commonly referred to as coal ash, are the residual materials after burning coal for electricity generation. CCRs include fly ash, bottom ash, boiler slag, and flue gas desulfurization (FGD).[67] CCRs are one of the largest waste streams in the United States and typically contain several metals, including As, Se, Hg, and Cd.[67,68] Approximately 56% of the CCRs generated are disposed of either in liquid form in surface impoundments or solid form in landfills, 37% are beneficially used, and 7% are used as minefill.[68] CCRs are currently generated in 45 states, with the largest amounts being generated in Kentucky, Texas, and Indiana.[68] Oregon, California, and Hawaii generate the least amounts of CCRs, thus a more locally abundant and cost-effective source may be available in these states.[68] Application of one CCR in particular, fly ash, has been shown to improve textural composition, which can enhance soil water retention capacity, improve aeration, and provide essential plant nutrients such as B, Se, and Mo for plant and/or animal nutrition. Fly ash can also increase soil pH and buffering capacity to counter soil sodicity.[69,70] The composition of the fly ash may differ due to the inherent variability of the chemical composition of the coal combusted however.[71,72]
5.2.2. Cement kiln dust (CKD)
Cement kiln dust (CKD) is a fine-grained, solid, highly alkaline waste similar to Portland cement that is removed from cement kiln exhaust gas by air pollution control devices. The physicochemical characteristics of CKD for their beneficial use depend on the method of dust collection employed at the facility.[74] CKD contains free lime with higher concentrations found in the coarser particles captured closest to the kiln. Finer particles exhibit lower free lime, but higher concentrations of sulfates and alkalis.[74] CKD can also contain trace amounts of Cd, Pb, Se, and radionuclides, thus fully characterizing this material is recommended before use. EPA has categorized CKD as a special waste, resulting in a temporary exemption from federal hazardous waste regulations under Subtitle C of Resource Conservation and Recovery Act (RCRA). EPA is currently developing standards for the management of CKD and proposed Subtitle D (non-hazardous solid waste) regulations.[75]
5.2.3. Lime kiln dust (LKD)
Lime kiln dust (LKD) is physically similar, but chemically different, to CKD. Its physicochemical properties vary most greatly depending on whether LKD is generated from the manufacturing of high-calcium lime (e.g., chemical lime, hydrated lime, or quicklime) or dolomitic lime.[74] The type of lime manufacturing dictates the concentrations of free lime and magnesium found in LKD. LKD generally has higher concentrations of free lime than CKD.
5.2.4. Red mud
Red mud is a waste by-product from alumina production using bauxite ores via the Bayer process. In the United States, bauxite is sourced from surface mines in Arkansas, but most bauxite used in the United States are imported.[76] For context, each metric ton of alumina produced generates 0.8–1.5 million metric tons of red mud (or approximately 35–40% of the processed bauxite ores results in a waste by-product).[77] Red mud varies in physical, chemical, and mineralogical properties as a result of different ore sources and refining processes used and therefore must be characterized before application. It consists of fine particles, high alkalinity (pH 10–12.5), high Fe content (30–60% of Fe2O3 by weight), and some metals (As and Cr).[76] Red mud has been beneficially used as an amendment in wastewater treatment for the removal of metals and metalloids, inorganic anions (e.g., nitrate, phosphate, fluoride, phosphate), and organics. Red mud has also used as a component of construction materials (e.g., clay, glass, brick, aerated concrete blocks); as a filler in road bases, mining sites, and in the manufacturing of polyvinyl chloride (PVC) plastic; in the treatment of waste gas containing S; and as a coagulate, adsorbent, and catalyst for various industrial processes including hydrofracking.[77] Red mud as a soil amendment has shown a reduction in metal mobility, and a low risk of metal remobilization associated with soil pH increase,[78] including decreased plant bioavailability.[79-81]
5.2.5. Agricultural limestone
Agricultural limestone is calcium carbonate (CaCO3) and may be referred to as Ag lime, garden lime, agricultural lime, and liming. It is an aggregate that has a similar chemical makeup and fineness of ground limestone. The fineness of lime correlates to how quickly the limestone will react with acids in the soil to increase pH. Because of its fineness, most agricultural limestone is used as a fertilizer to stabilize acidity in soil. Agricultural limestone is also used in coal burners at power plants to reduce air pollution emissions. Agricultural limestone has been proven to be effective in reducing Cd, Cu, Cr, Hg, Ni, Pb, and Zn leaching in soil.[22,82-87]
5.2.6. Lime-stabilized biosolids
Lime-stabilized biosolids result from the secondary treatment of municipal or industrial biosolids through the addition of quicklime or calcium hydroxide (hydrated lime).[88] The addition of lime raises the pH to a level as high as 12.4, at which the combination of lime and high temperatures destroy the cell membranes of any pathogens.[89] The high pH level of lime-stabilized biosolids causes water-soluble metal ions (except for Mo and Se) to convert to water-insoluble metal hydroxides that precipitate from the soil solution, thereby reducing their mobility and bioavailability.[88] Lime-stabilized biosolids has been shown to reduce the metal extractability and phytoavailability of Cd, Pb, and Zn in contaminated soils from smelter sites.[90] In general, few studies are available in the literature where lime-stabilized biosolids are used to immobilize metal-contaminated soil or sediment.
5.3. Mineral or inorganic waste by-product amendments
Waste by-products with mineral properties include iron/steel slag, phosphates, gypsum, and natural or synthetic minerals like leonardite and zeolite. These by-products are generated from a variety of industrial sectors including steel manufacturing, aluminum manufacturing, and coal combustion for electricity generation. The addition of amendments with inorganic/mineral by-products increase sorption sites for metal ions and can improve the physical quality of soil. A few of the waste by-products identified in the literature as alkaline by-product amendment materials are also inorganic materials, including red mud (Fe-rich), CKD, and CCR (e.g., fly ash and phosphogypsum). The composition of these by-product amendments varies considerably, and the immobilizing effect is mostly due to Al, Fe, and Mn oxides; phosphates; silicates; and alkaline materials.[91]
5.3.1. Slag
Slag is a broad term that covers all waste products resulting from the ore-separation process. Its chemical properties depend on the manufacturing and solidification process that has been used for refining metals. Slags are generally categorized as ferrous (e.g., Fe and steel) and non-ferrous (e.g., Cu, Pb, and Zn), and have mainly three types that are beneficially used: Fe blast furnace slag, basic oxygen furnace (BOF) steel slag, and electric arc furnace slag. Slag is rich in P and calcium phosphate and has been used since 1927 as agricultural soil amendments.[92] Due to the high fraction of calcium silicate minerals, slags are extensively used as a soil liming agent and are nearly as effective in neutralizing soil acidity as agricultural limestone.[93]
5.3.2. Steel shot
Steel shot refers to spherical grains of molten steel used to shape metal surfaces.[94] The particle size of steel shot varies, and the rate of application can have a significant impact on bioavailability of Cd and Zn.[94] Larger particle sizes were found to be less effective in reducing Cd and Zn uptake by plants compared to finer particle sizes.[95] Steel shot readily corrodes and oxidizes to form several Fe oxides and Mn oxides that may coat soil particles to create a large surface area for reactions. Single applications of steel shot, separately and in combination with beringite, have resulted in reduced Cd and Zn mobility.[94,96,97] The combination of beringite (5% wet weight [w/w]) with steel shot was found to be more effective in decreasing extractable metals and As.[31] Muller and Pluquet[98] found that treatment of Cd- and Zn-contaminated soil with bog iron ore and native steel shot caused a small increase in extractable Zn. In general, metal mobility and plant bioavailability in steel shot–treated soil may be controlled by Mn oxides.[99]
5.3.3. Beringite
Beringite is a modified aluminosilicate that originates from the fluidized bed burning of coal refuse in the former coal mine of Beringen in northeast Belgium. It is a strongly metal-fixing substance, relying on chemical precipitation, ion exchange, and crystal growth for metal immobilization.[100,101] Beringite amendments can increase pH and lead to dissolution of organic material, which may lead to unintended consequences. For example, Cu has a strong affinity to adsorb to soluble OM, thus treatment with beringite of Cu-contaminated soil is not suitable for immobilizing Cu.[102]
5.3.4. Foundry sands
Foundry sands are a by-product of the metal-casting industry. Approximately 6–10 million tons of spent foundry sands are generated annually, with less than 15% currently recycled.[103] Foundries reuse spent sands until the heat and mechanical abrasion renders the sand unsuitable for use in casting molds. The majority of spent sands are landfilled. Foundry sand has been beneficially used in the construction sector and as a soil amendment, but limited results were identified with respect to treating metals in contaminated soil and sediment. Spent foundry sands may contain metals and other contaminants such as cyanide, fluoride, and phenols; a full metals analysis should be conducted to fully characterize the by-product prior to use.
5.3.5. Coal combustion residuals
See the description of CCRs under the “Liming/Alkaline Waste By-Product Amendments” section (Section 5.2.1).
5.3.6. Phosphorus minerals
Phosphorus comes in two general categories: sparingly soluble forms (e.g., phosphate rock, synthetic apatites) and soluble forms (typically present in commercially available fertilizer products and phosphoric acid) and occurs in many minerals, of which apatite is the most abundant. Phosphate rock is a naturally occurring mineral containing calcium phosphate (Ca3(PO4)2). Calcium phosphate is highly insoluble in water, making the phosphorus not readily available to plants.[104,105] Phosphate rock and phosphate-based compounds are proven amendments for the immobilization of Pb-contaminated soil through the precipitation of pyromorphite minerals.[89,105-107] They have also been used specifically for Zn, Cu, and Cd immobilization via surface adsorption and complexation mechanisms.[31,46,82,86,89,107-110] Struvite (also referred to as monoammonium phosphate—MAP) is a phosphate mineral, chemically equivalent to magnesium ammonium phosphate hexahydrate (NH4MgPO4.6 H2O). Struvite occurs in sewage and wastewater treatment, as well as during the degradation of manures, and can lead to operational problems by forming a scale on belts, centrifuges, and pumps and can clog anaerobic digesters. Struvite can be recovered from waste streams, and has the potential for beneficial use as a fertilizer or soil amendment. Struvite solubility is low in water; however, it has been shown to dissolve slowly over time in soil environments.[111]
5.3.7. Gypsum
Natural gypsum is mined from geologic deposits, whereas synthetic gypsum is produced from FGD systems at electrical power plants. It is also generated through various sulfuric acid neutralization processes during the manufacturing of P fertilizers. Several researchers have applied gypsum to effectively immobilize Al, Cd, Cu, Pb, and Zn.[86,112-116]
5.3.8. Phosphogypsum
Phosphogypsum (PG) is calcium sulfate and a by-product of phosphoric acid production from phosphate rock. During PG production, naturally found radium within the phosphate rock exhibits radioactivity after reaction with sulfuric acid.[76,117] Therefore, EPA has banned the use of most applications of PG with radium-226 concentration greater than 10 picoCurie/gram. However, PG below this threshold can be beneficially used as road pavement, a soil conditioner, cover for landfills, roof tiles, and in artificial reefs and oyster beds to repopulate the marine environment. PG and red gypsum applied to acidic soil contaminated with Cu, Pb, and Cd showed that a 1% w/w amendment of either type increased the soil’s retention of all three metals. The study reported the highest reduction (98%) of Pb compared to Cu and Cd, which could be due to the formation of Pb minerals.[34]
5.3.9. Water treatment residuals
Drinking water treatment plants generate by-products in the form of amorphous masses of Fe and Al hydroxides; sediment and humic substances removed from raw water (e.g., flocculates, precipitates, fine clays, silts, and organics); and traces of coagulating agents (e.g., alums and other chemicals) used in the water treatment process.[118] Few studies have investigated the use of water treatment residuals (or alum sludge) for immobilization of metals in soil/sediment.[119-123] However, Fe-rich water treatment residuals could be appropriate to improve soil texture and treat soils contaminated with both As and cationic metals.[124] Water treatment residuals have a large surface area, and are highly reactive with increased sorption capacity. Water treatment residuals have been effective in raising pH and acting as a sorbent for excess P,[11] and other contaminants of concern that include As, Cu, Cd, Cr, Hg, Pb, Ni, Se, and Zn.[118,120,123,124]
Additional waste by-product amendments that are less frequently used include Alperujo, spent mushroom substrate, silkworm excrement, vermicompost, palm oil waste product, sugarcane filter cake, bagasse, sugar foam, sugar beet lime, wood ash, seashell grit, sea food, and meat processing by-products along with targeted contaminants, immobilizing mechanisms, and the associated limitations with their application. Details on field applications of these waste by-product amendments are provided in Table S3. The amendments mentioned thus far have chemical and physical properties that can assist in controlling metal availability and mobility in soil/sediment. As in all cases of remediation, the soil/sediment chemistry must be well understood to appropriately select the amendment materials for application, either as single amendments or in combination, to achieve the desired result.
The quantity of amendment needed is determined on a case-by-case basis and primarily depends on the application rate. Assessing the application rate is important because sustained changes in microbial structure, biomass, and function occur with multiple amendment applications.[125] Other factors that impact amendment selection are availability and source location and costs. Qualitative information on sources and availability throughout the United States, a general rating of costs (e.g., free, low, high), and limitations and unintended consequences of each amendment are included in Table S3. Certain amendments may have higher costs associated with them because they are commercial products (e.g., Bauxsol™) or the result of competing markets (e.g., municipal composting of yard and wood waste). Transportation costs and emissions are associated with all by-products, thus distance to the source must be considered. Characteristics of the by-products affecting transportation logistics and cost include water content (e.g., adds extra weight and may require special vehicle containers); odor (e.g., unpleasant to thruway residents); and particle size (e.g., finer materials will require a completely enclosed vehicle). Site-specific transportation factors include the presence of access roadways (e.g., residential vs. highway vs. unpaved roads) and their suitability for handling large loads. In general, transporting waste by-products from within 200 miles of a project site is considered economically viable.[11] Beyond that, rail hauling is a potentially expensive, but alternative option. Costs on a per-volume or per-area basis vary widely across the amendments. Cost considerations should cover availability and quantity needed, distance to/from the source (transportation and vehicle/equipment requirements), and onsite storage. For the most part, waste by-product amendments are available throughout the United States at low or no cost.
Storage of the by-products prior to use should also be considered, particularly if the by-product has the potential to generate odors or fugitive dust emissions or will become unstable when left uncovered and unmanaged. Seasonal temperature and precipitation may affect onsite storage decisions as well. For example, by-products with high moisture contents such as biosolids or pulp mill sludges can become anaerobic, decompose, and generate odors. These adverse effects may be higher in hot, humid summers compared to a colder winter.
5.4. Emerging waste by-products as amendments
The classification of amendments as emerging is somewhat subjective, but is mostly based on limited laboratory and field studies compared to more commonly used amendments (e.g., biosolids, CCR, CKD) and whether or not the amendment was identified in the U.S. EPA report entitled, The Use of Soil Amendments for Remediation, Revitalization, and Reuse.[11] The majority of emerging amendments identified in this section fall under the agricultural umbrella. The functional groups present in biomass molecules are known to have an affinity for metal complexation[126] and are a natural fit for metal treatment. These by-products typically undergo some form of chemical or physical modification to make them more suitable as an amendment (e.g., drying, grinding, composting, sieving, or pyrolysis in the case of biochar), but some may be directly applied. The biochar as an amendment to improve soil health and the environment has been highly utilized.[127] Only those studies that presented information pertaining to the use of non-municipal, non-hazardous waste by-products as soil/sediment amendments are included in this section. Studies were retained if an existing waste by-product was included in a study as a component of an amendment mixture.
Table 3 presents agricultural waste by-products that have been used to treat metal contamination in water, soil, or sediment in a laboratory or small field-scale applications. Few studies demonstrating field applications of emerging waste by-product amendments for metal treatment in soil/sediment were identified in the literature review. Certain agricultural waste crops used to treat metals, but not typically found in the United States, include black gram (a common Indian pulse), jatropha (a flowering plant used for biofuels), and palm oil manufacturing by-products.[161] Other waste by-products classified as emerging include digestates from anaerobic digestion, red gypsum (a by-product of titanium dioxide production), and various by-products from the seafood processing industry (e.g., fish bone char, crab, oyster, mussel shells, and seashell grit). An extensive overview of emerging waste by-products, including the general mechanisms (e.g., sorption, precipitation, complexation) for metal immobilization observed are provided along with the general availability of the waste by-product within the United States, benefits, and unintended consequences is presented in Table 4
Table 3.
Emerging agricultural waste by-products as soil amendments.
| Agricultural waste by-product | References |
|---|---|
| Rice residuals (e.g., bran, husk) | [127,128,129,130,131,132] |
| Wheat residuals (e.g., bran, husk) | [133,134] |
| Oat biomass | [135] |
| Saw dust | [136] |
| Coconut shells and fiber pith | [137] |
| Seaweed | [138] |
| Nut shells from walnuts, peanuts, hazelnuts, and coconuts | [139,140,141,142,143] |
| Cotton seed hulls and cotton stalks | [144] |
| Waste tea leaves | [145] |
| Spent mushroom substrates | [146] |
| Olive oil extraction wastes (i.e., alperujo) | [147,148] |
| Sugarcane and beet sugar processing by-products (e.g., pulp, filter cake, bagasse, lime, vinasse, sugar foam [SF], boiler ash) | [143,149,150,151,152,153,154,155,156,157] |
| Apple cores and peels | [158] |
| Banana peels | [159] |
| Orange peels | [159] |
| Soybean hulls | [143] |
| Sunflower stalks | [144] |
| Coffee grinds | [160] |
Table 4.
Overview of emerging waste by-products as amendments.
| Waste by-product category and amendment |
Source | Availability and general costs |
Metal uptake | Benefits of using | Limitations and unintended consequences |
Targeted metal(s) | |
|---|---|---|---|---|---|---|---|
| Organic | Digestates (from anaerobic digester) | Anaerobic digestion of organic wastes (e.g., biosolids) | • Availability dependent on the location of anaerobic digesters | Presumed to be similar to biosolids due to the high organic matter content–sorption, complexation of the metal fractions associated with organic matter, metal oxides, or carbonates53 | • High nutrient content | • Relatively new material and not well characterized; may contain metals | No studies were identified through the literature review that specifically investigated immobilization of metals in soil or sediment |
| • Relatively new product, and costs are undetermined | • Source of OM | • Not regulated | |||||
| • Not routinely treated for pathogen reduction, although digestion temperatures are adequate to kill off pathogens | |||||||
| • Potential odor issues | |||||||
| • Possible leaching of phosphorous (P) | |||||||
| Organic | Biochar (also charcoal and activated C) | Pyrolysis or gasification of biomass (e.g., wood, straw, and tree bark) | Approximately 20+ biochar manufacturers across the United States162 | Adsorption due to large surface areas | • High surface area and cation exchange capacity (CEC); most effective for organic compounds | • Low-temperature pyrolyzed biochars may degrade quickly and remobilize any sorbed metals | As, cadmium (Cd), chromium (Cr), copper (Cu), nickel (Ni), lead (Pb), and zinc (Zn) |
| • C-rich | • Application of biochar increases OM and may either increase or decrease dissolved organic carbon (DOC); if it increases DOC, metals may mobilize (e.g., arsenic [As]) | ||||||
| • Attracts microbes and beneficial fungi | |||||||
| • Has a long life in soils compared to fertilizers | |||||||
| Organic | Rice residues biochar (e.g., hulls/husks, straw, bran) | By-product from rice processing | • Major rice-producing states are Arkansas, California, Louisiana, Mississippi, Missouri, and Texas163 | • Adsorption | • Husks have low solubility in water, good chemical stability, and structural strength as a result of a high silica content | See Biochar | Pb, Cd, Zn, Ni, and As |
| • More than 19 billion pounds of rice are produced, but the amount of wastes generated were not determined | • Complexes and chelates are formed | • See Biochar | |||||
| Red gypsum (or titano-gypsum) | Titanium dioxide (TiO2) production (pigment industry) | Of 142,000 metric tons (MT) of raw material processed, 70,000 MT of red gypsum produced | Adsorption | • Rich in iron (Fe) oxides | Some variability exists due to raw material properties | As, Cu, Pb, Cd, Zn, and Ni | |
| • Useful as a soil amendment | |||||||
| Organic and liming/alkaline | Alperujo | Olive oil extraction processes | • Olive oil is produced in California, Arizona, Texas, Georgia, Florida, Oregon, and Hawaii164 | Free ions can complex and co-mobilize with organic ligands during leaching events due to the addition of OM | • Addition of organic matter and potassium (K) | • Not widely available in the United States | As, Cd, Cu, Pb, Zn, and Mn |
| • Annual amount of waste generated was not determined | • Provides nutrients (C, nitrogen [N], and P) | • May mobilize As in multicontaminant sites as a result of increases in soil pH | |||||
| • Lower levels of metals than biosolids or municipal solid waste (MSW) compost | |||||||
| • Slow release of nutrients as evidenced by low mineralization rate165 | |||||||
| Organic | Spent mushroom substrate (compost) | Mushroom crops | Approximately an equal ratio of spent mushroom substrate is generated per weight of mushrooms ready for consumption | Adsorption | • Addition of nutrients and OM | • May require additional processing after composting to obtain the desired and most effective particle size | Cd, Pb, Cr, and As |
| • High pH buffering capacity due to the addition of lime during composting of the substrate | • Competing uses (e.g., crop production, reuse in the cultivation of mushrooms, animal and fish feed, pest management) | ||||||
| • Generates relatively high volumes of waste | |||||||
| Organic | Silkworm excrement | Silkworm culture | • China leads the world production; approximately seven companies in the United States operate silkworm farms166 | Sorption | Addition of nutrients and organic matter | Unknown at this time | Cd and Pb |
| • Information was not obtained regarding annual quantities of excrement generated or cost | |||||||
| Organic | Vermicompost | Vermicomposting of various organic wastes such as vegetable or food waste using worms | • Large quantities available near large-scale vermicom-posting facilities | Adsorption by negatively charged functional groups | • Produces nutrient-rich humus | Potential metal contamination depending on feedstock (e.g., pig manure, sewage sludge, fly ash, and cow dung) | Cd, Pb, Zn, and Cu |
| • Relatively low cost | • Addition of nutrients and organic matter | ||||||
| Organic | Palm oil waste by-products (e.g., palm kernel pie, boiler ash, empty fruit bunches) | Palm oil manufacturing | Palm oil by-products are not available in the United States; would need to be sourced internationally | Adsorption | Fertilizer | Unknown at this time | Cd and Zn |
| Organic and liming/alkaline | Sugarcane filter cake | Residue from sugarcane juice filtration | • Sugarcane is produced in Florida, Louisiana, Hawaii, and Texas, with Florida producing the most167 | Adsorption | • Addition of P and organic matter | Unknown at this time | Cd and Zn |
| • By-products are generally free | • Use of by-products would offset effects from field burning (where applicable) | ||||||
| Organic and liming/alkaline | Bagasse | By-product after crushing sugarcane or sorghum stalks to extract their juice | • See Sugarcane filter cake | Adsorption | Use of by-products would offset effects from field burning (where applicable) | • Potential for fugitive dust | Cd and Zn |
| • Used as a fuel source for sugar mills, so its availability for other uses is limited | • Unpredictability in annual generation due to weather and economics | ||||||
| Organic and liming/alkaline | Sugar foam (SF) | By-product from sugar manufacturing | See sugarcane filter cake | • Adsorption | • Addition of CaCO3 | • Potential for fugitive dust | As, Cd, thallium (Tl), Zn, Mn, Cu, aluminum (Al), and Fe |
| • Formation of Al-hydroxy polymers | • Can increase pH | • Unpredictability in annual generation due to weather and economics | |||||
| Organic and liming/alkaline | Sugar beet lime | Spent lime from the purification of sugar from sugar beets or sugarcane (lime is added to neutralize organic acids present in the plant material) | • Grown in 5 regions encompassing 11 states, primarily in the western United States167 | • Adsorption | • Increases pH | • Potential for fugitive dust | Cu, Zn, Pb, and Cd |
| • By-products are generally free | • Chelation, complexation with carboxyl groups | • High calcium (Ca), magnesium (Mg), and K | • Relatively high water content, which affects transportation costs | ||||
| • Can contain organic matter | • Unpredictability in annual generation due to weather and economics | ||||||
| • Fine particle size | |||||||
| Liming/alkaline | Wood ash | Wood-fired utilities | • Available in small to moderate amounts from wood-fired utilities | Sorption | • Increases pH | • Highly variable content | No studies were identified through the literature review that specifically investigated the immobilization of metals in soil or sediment |
| • Materials are generally free | • Source of Ca, Mg, and K | • May contain contaminants if other fuels (e.g., tires or waste oil) are co-combusted | |||||
| • May have dioxin and should be confirmed through testing | |||||||
| • Lime equivalent will vary by burn temperature and age of material | |||||||
| Liming/alkaline | Seashell grit | Seafood processing | Available near coastlines with fishing or seafood processing operations; low costs | Adsorption | • pH increase and addition of Ca | None identified | As, Cu, and Zn |
| • Used in horticulture and poultry industry | |||||||
| Odor? | |||||||
| Liming/alkaline | Chitin, chitosan (as found in the exoskeletons of shellfish and crustaceans, as well as lobster, mushrooms, and bacteria) | Seafood processing | • Seasonally available near coastlines with fishing or seafood processing (canning) industries | Adsorption | • Source of Ca from CaCO3 | None identified | Al, As, Cd, Cr, Cu, Fe, mercury (Hg), manganese (Mn), Ni, and Zn |
| • Presumed low costs | • Slow release of N, P, and Mg | ||||||
| • May contain some K and chitin | |||||||
| • Second most abundant natural biopolymer (after cellulose) and can be assumed to be of plentiful supply | |||||||
| Odor | |||||||
| Mineral/inorganic | Hydroxyapatite (phosphate in the form of apatite from fish bones) | Seafood processing | Available near coastlines with fishing or seafood processing operations; presumed low costs | Precipitation, solid solution | • Addition of P to the soil | None identified | Pb in soil, numerous other metals in aqueous solution |
| • Used as a fertilizer in land application and horticulture | |||||||
| Odor | |||||||
| Mineral/inorganic | Bone char, bonemeal, bonemeal biochar (finely ground, poorly crystalline apatite, Ca10(PO4)6OH2) | Meat processing (e.g., cow bones) | Readily available near animal farms | • Adsorption | Free of metal and other types of contaminants | See Biochar | Cd, Pb, and Zn168 |
| • Formation of metal phosphates | |||||||
5.5. Candidate waste by-products for further assessment
Identifying amendment substitutes, for virgin materials, relies on identifying the physicochemical properties of the materials and how they compare and contrast with virgin materials. The substitution of waste by-products for virgin-based amendments certainly has environmental and economic benefits that need to be further investigated prior to their successful applications. Using the North American Industry Classification System (NAICS), we have identified several waste by-products from various industrial sectors, including even those with limited or no application for metal immobilization. In order for waste by-products to be eligible for further assessment, they must be non-hazardous and non-MSW, and must either be an emerging amendment or not currently used as an amendment for soil/sediment metal contamination as presented in Table S4. This section presents the data collection focused on peer-reviewed literature and government (e.g., U.S. EPA) publications for selected physical and chemical properties of amendments that have been reported to contribute in their successful application, or effectiveness, in immobilizing and/or reducing the bioavailability of metals in soils and sediments. A vast array of additional information on these amendments properties is anticipated to be available outside of the realm of metal remediation literatures. Physicochemical properties of waste by-products such as pH, particle size, surface area, OC, and OM have been generally used in the literature as a baseline to make a decision for further assessment. In general, based on the data available from the literature, singling out one physical or chemical property of an amendment as being the driver for metal immobilization is challenging. Therefore, a combination of these amendment properties is most likely responsible for metal reduction in soil and sediment. Additionally, the environmental characteristics are equally significant in determining the effectiveness of a specific amendment (Table S1). The candidate waste by-products categorized as emerging are presented in Table 5
Table 5.
Detailed study focused on candidate waste by-products.
| Lab or field |
Waste by-product amendments used |
Description of tested medium and site |
Targeted metals |
Successful amendment(s) or blend(s) (final application rate) |
Maximum Metal reduction/change |
Maximum plant success (post- treatment) |
Findings | References |
|---|---|---|---|---|---|---|---|---|
| L | Phosphogypsum (pH = 3.8) | Contaminated soil (central Spain); pH = 5.45 | Arsenic (As), cadmium (Cd), and thallium (Tl) | SF2 = 1% (adds 25.5 kg calcium [Ca]) | As: 26% more retained (pretest: 616.2 mg/kg) | Not presented | Enhanced immobilization, particularly for As and Cd and Tl to a lesser extent. Presence of Cd may favor As adsorption due to formation of aluminum (Al) hydroxy polymers or reprecipitation of new carbonate minerals | [169,170] |
| Sugar foam (SF) (pH = 9.3) | Cd: 49% more retained (pretest: 568.4 mg/kg) | |||||||
| Tl: 42% more retained (pretest: 693.2 mg/kg) | ||||||||
| F | Sewage sludge (SS)/paper mill (PM) residues/aluminum plant sludge blend; pH = 8–10 | Acid mine soils at the depleted copper mine (northwest Spain); pH = 3.3 | Chromium (Cr), copper (Cu), nickel (Ni), lead (Pb), and zinc (Zn) | SS/PM residues (forest and sludge field site: 297 metric tons/hectare (ha) of SS + PM) | Cr: 41% of 130 mg/kg | Increase bacterial and fungal abundance and microbial functions | Addition of the sludges and residues recovered bacterial and fungal abundance; however, the high Cu and Cr concentrations still negatively impacted microorganism activity and plant recovery. Activity of the acid phosphatase may have been negatively affected due to the feedback inhibition by inorganic phosphate usually found in SS. Sites treated with sludges decreased the specific activity of all enzymes involved in carbon (C) and nitrogen (N) cycle indicating that their microbial community is characterized by higher C and N use efficiency than unamended soil | [54] |
| Cu: 90% of 644 mg/kg | ||||||||
| Ni: 69% of 14.64 mg/kg | ||||||||
| Pb: 43% of 21,99 mg/kg | ||||||||
| Zn: 4% of 113 mg/kg | ||||||||
| L | Lime-stabilized biosolids (pH = 12.3), rock phosphate (pH = 7), and anaerobic biosolids (pH = 7.1) | Contaminated soil from Zn and Pb milling and smelting operations in Oklahoma; pH = 6.4–6.88 | Cd, Pb, and Zn | Lime-stabilized biosolids (LSB) 100 g/kg soil | Post-90 days | LSB significantly reduced the concentration of Cd and Zn in lettuce | The largest reductions in metal extractability and phytoavailability were realized using LSB; however, this ability was lost when soil was acidified to pH <6 | [89] |
| Phytotoxic Zn: 86% of 1,188 mg/kg | ||||||||
| Cd: No values given | ||||||||
| Zn: No values given | ||||||||
| F | Agricultural limestone (pH = 7.3), mineral rock phosphate (pH = 6.84–7.11), and diammonium phosphate (DAP) (pH = 7.45–8.02) | Surface soil (<20 cm) with elevated residual metal concentrations from an inoperative smelter site in northeastern Oklahoma; pH = 6.97 | Cd, Pb, and Zn | DAP 10 g/kg soil | Post-6 months | Not presented | DAP was the most effective treatment for immobilizing heavy metals | [82] |
| Cd: 94.6% | ||||||||
| Pb: 98.9% | ||||||||
| Zn: 95.8% | ||||||||
| L | Alperujo compost biochar | Contaminated soil (top 10 cm; <2 mm fraction) from sporadically vegetated part of the La Mina Monica mine site area, Spain | As, Cd, Cu, Pb, and Zn | Compost (C) 10 v/v | (BC) Reduction in pore water: | (C) Germination success increase 55% | The greatest reduction of metal concentrations in pore water was achieved by biochar; however, application of both alperujo compost and biochar increased the potential for As leaching. Compost increased plant growth, and compost and biochar together decreased toxicity the most | [147] |
| Biochar (BC) 10% v/v | As: 80% (increased mobilization) | (C) Root length increase 52% | ||||||
| C+BC 5% v/v each | Cd: 98% of ~130 μg/L | (C + BC) Toxicity decrease ~50% | ||||||
| Cu: 98% of ~1,150 μg/L | ||||||||
| Pb: 95% of ~95 μg/L | ||||||||
| Zn: 95% of ~7,500 μg/L | ||||||||
| F | Hydroxylapatite (pH = 7.3) | Sediments (top 20 cm; <2 mm fraction) were contaminated due to past and present non-ferrous metal processing and smelting activities; pH = 7 | Zn, Cd, Cu, Pb, and As | TBS, 5% DW | Cd: 81% of 0.64 mg/kg | Species richness reduction: | The incorporation of TBS into the metal-contaminated sediment decreased the extractable fraction of Cd and Zn. In contrast, the hydroxyapatite did not decrease Cd and Zn extractability. TBS was effective in Cd and Zn immobilization, did not cause any nutrient imbalance or sediment ecotoxicity, and improved the bacterial activity | [171] |
| Thomas Basic Slag (TBS) (pH = 7.7) | Cu: 42% of 0.12 mg/kg | Before = 21 | ||||||
| After = 14 | ||||||||
| 33% reduction | ||||||||
| F | Biochar–wheat straw (pH = 10.4) | Rice paddy topsoil contaminated by emissions from a metallurgical smelter; pH = 5.36 | Cd and Pb | Biochar (40 t/ha) | Post-2 years | Cd% reduction shoot: 27.0–75.0% | By-product successfully used to increase pH, total organic carbon (TOC), and decreased extractable Cd over a 3-year period. Cd was reduced in rice plant tissues, while Pb reduction was found only in roots. Analysis indicated that Cd and Pb had bonded with mineral phases of Al, iron (Fe), and phosphorous (P) on and around the inside of the biochar particles. Immobilization of Cd and Pb also occurred to cation exchange on the porous C structure of the biochar particles | [172] |
| Cd: 46% of 2.72 mg/kg | Cd% reduction root: 29.1–57.8% | |||||||
| Pb: 35% of 63.44 mg/kg | Cd% reduction rice plant uptake: 27.5–67.33% | |||||||
| Pb% reduction rice plant uptake: 27%–69% | ||||||||
| F | Biochar–wheat straw (pH = 10.4) | Contaminated rice paddy topsoil pH ranged from 5.01 to 6.31 | Cd | Wood biochar (40 tons/ha) | Cd: 92% reduction reported for Cd (pretreatment range 0.31–3.13 mg/kg) | Rice grain Cd% reduction: 42–48% | Reduced Cd uptake by rice and immobilized Cd and Pb | [173] |
| F | Phosphate via fish bones (pH not reported) | Soil-contaminated from former chemical distribution facility and metals scrap yard (U.S. Superfund site); pH = not reported | Pb | Not presented | Not presented | Not presented | Immobilization of Pb achieved through the precipitation of pyromorphite (a mineral consisting of a chloride and phosphate of Pb) | [174] |
| F | Biosolids, agricultural lime, sugar beet lime, lime kiln dust (LKD); pH = 6.39 | Fluvially deposited mine tailing sediment (Colorado); pH = 4.9–5.3 | Zn and Pb | Biosolids (BS) + LKD | (LKD + BS): Zn: 99% of 970 mg/kg | Post: (LKD + BS) 95% cover | Soil pH increased and extractable metals decreased in lower soil horizons. Plant response in the field study was disappointing. Use of LKD + BS showed lush growth after an initial period of no growth. Response to in situ amendments may vary across large-scale restoration efforts | [55] |
| LKD only | (LKD): Cd: 100% of 7.5 mg/kg | |||||||
| Each added to lime equivalent of 224 mg/ha CaCO3 | ||||||||
| L | Fresh alperujo (pH = 5.43); sugar beet lime (pH = 9.04); composted biosolids (pH = 6.93) | Trace element polluted soil near a mine (acidic and alkaline soils tested); pH = 4–7 | Cd, Cu, Mn, and Zn | Alperujo separate applications of about 30,000 kg/ha | Soil A | Pre: – | Alperujo stimulated biological activity; both alperujo and sugar beet lime effectively immobilized metals | [148] |
| Cd: 0% of 0.4 mg/kg | Post: increase in microbial biomass | |||||||
| Cu: 57% of 3.5 mg/kg | % reduction:– | |||||||
| Mn: 20% of 200 mg/kg | ||||||||
| Zn: 6% of 75 mg/kg | ||||||||
| F | Seashell grit (pH = 6.47) (compared and in combination with biosolids, natural zeolite, and iron-activated zeolite) | Degraded topsoil surrounding copper smelter (Puchuncaví Valley, central Chile); pH = 6.3 | Cu, Zn, and As | Seashell grit (SG: 0.5 tons/ha) + Fe-activated zeolite (AZ: 1 ton/ha) | Post-14 weeks: | Rye grass post (SG, AZ, Z, and B): biomass/root cover 125–280% higher than reference soil | Eliminated phytotoxicity and bioaccumulation in plant tissues; reduced Cu and Zn, but not As solubilization in pore water | [56] |
| SG only: | ||||||||
| Seashell grit only (SG: 0.5 tons/ha) | Zn: 98% of 6.36 mg/kg | |||||||
| Note: Inclusion of biosolids had no apparent effect on metal reduction | SG + AZ: | |||||||
| Cu: 89% of 4.81 mg/kg | ||||||||
| As: 1.5% of 38.2 mg/kg | ||||||||
| L | Individual applications of vermicompost, sugarcane filter cake, palm kernel pie (compared to and in combination with lime, phosphate rock, and zeolite); pH range = 5.3–6.5 | Supernatant of amendment solutions from Cd-contaminated soils; pH = not reported | Cd | Vermicompost, sugarcane filter cake, palm kernel pie, zeolite, and lime | Best performance for adsorption was from both liming agents (lime and also the zeolite) | Not presented | Increased precipitation of Cd most successfully via liming agents (lime and zeolite) | [60] |
| All amendments 0.25 g of soil amendment and 25 mL of a synthetic Cd solution containing 0, 1.12, 5.62, 11.24, 16.86, 33.72, or 84.30 mg/L Cd | ||||||||
| F/L | Phosphoric acid | Contaminated hardrock mining site soils | Pb | Not presented | Not presented | Post-18 months: | Phosphate amendments resulted in overall bioavailability reduction | [175] |
| Bioavailability reduction: | ||||||||
| Pig 29% | ||||||||
| Rat 40% | ||||||||
| Human 69% | ||||||||
| In vitro (pH 1.5) 18% | ||||||||
| In vitro (pH 2.5) 69% | ||||||||
| F | Limestone | Contaminated hardrock mining site soils at Palmerton, PA “Revival Field”; pH = 6.25 | Cd and Zn | Biosolids compost application rate not presented | Post-5 years | Not presented | Biosolids compost resulted in a reduction of Cd- and Zn-extractable soil concentrations | [175] |
| diethylenetriamine pentaacetate (DTPA)–extractable reduction | ||||||||
| Biosolids compost | Cd 16.8% from 83.1 mg/kg | |||||||
| Zn 7.8% from 4940 mg/kg | ||||||||
| L | Individual applications of biochar–wood (pH = 10.9), crushed oyster shell (pH = 8.11), blast furnace slag (pH = 9.83), and fluidized-bed crystallized calcium (pH = 9.44) | Alluvial Cd-contaminated topsoil (0–20 cm), from illegal discharge of industrial wastewater (northern Taiwan); pH = 4.42 | Cd | Crushed oyster shell (OS) | Post-90 days | 58% reduction of Cd in rice shoots | Cd mobility and bioavailability reduced by all amendments | [69] |
| (Soil + 4% OS) | Cd: 35% reduction of 3.85 mg/kg | |||||||
| L | Red mud (pH = 11.12) | Sandy clay loam (top 30 cm, <2 mm fraction) from abandoned mining site of Baccu Locci (Villaputzu, Italy); pH = 6.84 | As (Cd, Cu, Pb, and Zn) | 3% w/w amorphous Al hydroxide (Al-OH) | As Al-OH ~65% | Plant growth | The adsorbed As fraction did not change in red mud-treated soil compared to control but decreased significantly in Fe-rich water and Fe2O3 soils (~27% reduction), and Al-OH soil (~65%). Plant growth, estimated by shoot dry weight, was significantly influenced by the amendments, and Al-OH was the best treatment followed by Fe-WTR | [176] |
| Hematite | Al-OH 2.5 and 3 times > control | |||||||
| Fe-rich water | Fe-WTR 1.8–2.5 > control | |||||||
| treatment residual (pH = 7.78) | ||||||||
| Amorphous Al hydroxide | ||||||||
| L | Cyclonic ash (norbergite) commercial Na-silicates | Soil (<2 mm fraction) contaminated by metal smelters in Flanders, Belgium; pH = 6.3 | Cd | Na silicate + cyclonic ash (CA + SIL) 5% CA +1% SIL | Zn 98% of 410 mg/kg | Post-28 days: | All tested amendments reduced Ca(NO3)2− extractable soil metal concentrations and reduced metal uptake in Agrostis capillaris seedlings. Cyclonic ash appeared efficient in both reducing oxidative stress in beans and Zn, Cu, and Pb uptake in grasses | [177] |
| Cd 95% of 5.6 mg/kg | Agrostis capillaris uptake reduction | |||||||
| Cu 88% of 5.2 mg/kg | Zn 93% of 5330 mg/kg DW | |||||||
| Pb 96% of 4.7 mg/kg | Cd 86% of 31.5 mg/kg DW | |||||||
| Cu 60% of 81.6 mg/kg DW | ||||||||
| Pb 82% of 72 mg/kg DW | ||||||||
| L | Marble sludge (pH = 8.5), compost (pH = 8.7), and bayferrox (pH = 7.6) | Contaminated soil (top 20 cm, <2 mm fraction) from the El Arteal mining district (Almeria, SE Spain) | As, Cd, Cu, Pb, and Zn | Marble sludge (MS) at 4 and 8% (w/w), compost (CM) at 2 and 6% (w/w), and synthetic Fe oxides (BF) at 1 and 3% (w/w) | (MS) Zn, Cd, Pb >90% | Highest root elongation index (reduction of toxicity to lettuce) | Amendments that raised the pH, especially MS, effectively diminished soluble heavy-metal concentrations; however, these amendments increased As concentration in lixiviates, encouraging the risk of the As dispersion. The amendment that fixed As, iron oxide, was not effective in diminishing soluble heavy-metal concentrations | [178] |
| Cu 50–60% | MS (8%) + CM (2%) | |||||||
| (BF) As 66% from 0.03 mg/L | Soils treated with Bayferrox, alone or mixed with MS or compost, were the most phytotoxic | |||||||
| L/F | Red mud (pH = 10.2) | Contaminated soils (top 23 cm, <2 mm fraction) adjacent to a decommissioned Zn/Pb smelter at Avonmouth in the United Kingdom; pH = 4.7 | Pb, Zn, Cd, and Cu | Red mud 5% | Post-21 months | Post-21 months | Field: Application of lime or red mud amendments had no significant effect on total metal concentrations in soil pot experiments. However, the 5% red mud treatment increased Cr concentrations from 45 mg/kg up to 135 mg/kg due to Cr in red mud | [78] |
| Cu, Zn, Cd, Cu – no reduction | average Festuca rubra yield 4,400% increase of 0.2 t/ha | |||||||
| Pot: reduction in pore water | ||||||||
| Pb 84% of 4.58 mg/L | ||||||||
| Zn 7% of 2.5 mg/L | Post: Red mud alone decreased uptake of Pb in Festuca rubra | |||||||
| Cd 95% of 6.11 mg/L | ||||||||
| Cu 37% of 2.58 mg/L | ||||||||
| Ni 97% of 0.3 mg/L | ||||||||
| L/F | Fly ash (pH = 13) and steel slag (pH = 13) | Soil (top 15 cm; <2 mm fraction) from paddy field in Shangba, China, contaminated by Dabao Mountain sulfide mining; pH = 4 | Cd, Zn, Pb, and Cu | Fly ash (FA40) 40 g/kg, steel slag (SS6) 6 g/kg, and steel slag (SS3) 3 g/kg | Post | Decrease of metal contents in leaf to stem ratio | The experiment indicated that the application of fly ash and steel slag increased soil pH from 4.0 to 5.0–6.4, decreased the phytoavailability of heavy metals by at least 60%, and further suppressed metal uptake by rice | [179] |
| (FA40) | (FA40) Cd 0.13 | |||||||
| Cd: 99% of 7.3 μg/kg | (SS3) Zn: 0.19 | |||||||
| Zn: >99% of 6.7 μg/kg | (SS3) Cu 0.40 | |||||||
| Pb: >99% of 57 μg/kg | (SS6) Pb 0.29 | |||||||
| (SS6) | ||||||||
| Cu: 89% of 6.3 μg/kg | ||||||||
| L | CaCO3 | Soil (top 14 cm, <2 mm fraction) contaminated by Zn industries in Prayon (Liège province, eastern part of Belgium); pH = 5.8 | Cd, Pb, and Zn | 25 g mass of CaCO3 (C) and iron grit (IG) to 500 g of contaminated soil and 250 g of washed sand | Reduction in flow-weighted mean concentrations | Bone meal reduced Pb concentrations in white lupin shoots by 74% | Cd and Zn leaching was reduced by all amendments mainly due to alkalinity increase. Pb leaching was strongly affected by DOC release. Therefore, bonemeal and manure treatments, which highly increased DOC concentrations in leachates, increased the flow-weighted mean Pb concentrations by 2.3 and 16 times, respectively. While iron grit induced strong Cd and Pb leaching reductions, this amendment doubled Cd and Pb concentrations in shoots of white lupin. Conversely, the addition of bone meal reduced Pb concentrations in shoots by 74%, probably because of organo-Pb complexes. Overall, the addition of CaCO3 offered the best compromise, as it successfully reduced both the leaching and the phytoavailability of the three considered metals | [180] |
| Iron grit, fly ash, manure, bentonite, and bone meal | (C) Cd 88.3% from 98 μg/L | |||||||
| (C) Zn 98.5% from 2875 μg/L | ||||||||
| (IG) Pb 83% from 15 μg/L | ||||||||
| L | Food waste compost (60% food waste and 40% sawdust) (pH = 7.76), market compost (50% food waste, 10% agricultural waste, 10% manure, and 30% lime), and zeolite (pH = 8.44) | Weathered tailings from abandoned mine site in South Korea; pH = 3.03 | Zn, Mn, Cr, Fe, Cd, and Al | Mixed with 200 g tailings: | Post-4 weeks: | Not presented | Both organic and inorganic materials were successful for increasing pH and metal immobilization, except in the case of As, where leaching potential increased up to 158%. With the exception of Market compost and Zeolite (MCZ), amendments increased leachability of Pb up to 43% | [181] |
| Reduction in metal leaching potential | ||||||||
| (MCZ) Market compost (50 g) + Zeolite (12.5 g) | Zn (MCZ) 91% | |||||||
| Mn (MCZ) 76% | ||||||||
| (Z) Zeolite (12.5 g) | Cr (Z) 53% | |||||||
| Fe (MCZ) 44% | ||||||||
| Cd (MCZ) 43% | ||||||||
| Al (MCZ) 24% | ||||||||
| L | Limestone waste, red mud, and furnace slag | Mine area soil (Korea); pH = 4.58 | As, Cd, Pb, and Zn | Limestone (LS), red mud (RM), and furnace slag (FS) applied to soils at 5% w/w | As (RM): 77% of 0.13 mg/kg | Bioaccessible fraction reduction in lettuce: | Fe-rich industrial by-products proved highly effective at stabilizing As and heavy metals, decreasing the levels of extractable contaminants. As a result, a corresponding reduction in the concentrations of these contaminants occurred in plant (lettuce). In addition, the reduction in extractable As and metals led to increases in microbial activity | [182] |
| Cd (RM): 98% of 0.959 mg/kg | As (FS) 46% | |||||||
| LS + RM and LS + FS at 2% w/w ratio | Pb (LS): 99% of 1.25 mg/kg | Pb (RM) 44% | ||||||
| Zn (LS+RM): ≤100% of 27.79 mg/kg | ||||||||
| L | Zero valent iron limestone (pH = 9.50), acid mine drainage treatment sludge (pH = 8.21), bone mill (pH = 6.69), and bottom ash (pH = 9.23) | Topsoil (<20 cm depth, <2 mm fraction) from contaminated sites in the Suseong gold mining area, Chungnam Province, Korea | Cd, Pb, and Zn | 2% (w/w); limestone (LS); bottom ash (BA) | Post – 40 days | BM lettuce shoot yield increase reduction in lettuce shoots | Ls and Ba were most successful in reducing metal extractability. With the exception of the BM treatment, lettuce shoot yields did not differ significantly among treatments. However, BA increases Cd concentrations in lettuce shoots compared to untreated soil | [183] |
| (LS) Ca(NO3)[2 extractability reduction | Cd (BA) ~69% | |||||||
| Cd 19.30% | Pb (BA) ~61% | |||||||
| Pb 37.43% | Zn (BA) ~58% | |||||||
| Zn 52.21% | ||||||||
| (BA) DTPA extractability reduction | ||||||||
| Cd 35% | ||||||||
| Pb 38% | ||||||||
| Zn 25% | ||||||||
| L | Bone mill (pH = 8.91), bottom ash (pH = 9.23), furnace slag (pH = 10.57), red mud (pH = 11.32), and plant species (Miscanthus sinensis and Pteridium aquilinum) | Mine tailings from the Suseong Pb/Zn mining area in Chungnam Province, Korea; pH = 7.64 | Cd, Cu, Pb, and Zn | Red mud 2% (w/w) and P. aquilinum (fern) | Post-90 days reduction in porewater | P. aquilinum planted tailings | The most significant reduction in heavy metal concentrations in pore water was observed in the RM-amended tailings for both plant species. In contrast, the BM application increased the concentrations of all metals evaluated in pore-water samples | [184] |
| Cd 99% | Decrease of bioavailability: | |||||||
| Cu 99% | Pb 34% | Furnace slag and M. sinensis reduced CaCl2-extractable heavy metals by 56–91%. Red mud and P. aquilinum treatment was the most effective at decreasing bioaccessible Pb, reducing it to 34% of the total Pb | ||||||
| Pb 98% | Mobility factor decrease: | |||||||
| Zn 99% | Cd 79–96% | |||||||
| Pb 77–91% | ||||||||
| Zn 77–96% | ||||||||
| N/A | 1. Zeolite | 1. Mine soils | 1. Zn, Pb, Cu, and Cd | 1. 0.5–5% by weight | 1. Labile and easily available fractions 42–72% | 1. – | Nanomaterials with high potentials for mine soil reclamation include zeolites, nZVI, iron oxide nanoparticles, phosphate-based nanoparticles, iron sulfide nanoparticles, and C nanotubes | [185] |
| 2. Zeolite | 2. Contaminated soil | 2. Hg | 2. 1–5% (g/g) | 2. – | 2. Plant uptake (rye and alfalfa) 58% in roots and 86% in shoots | |||
| 3. Zeolite | 3. Soil near Zn–Pb smelter | 3. Cd, Pb, and Zn | 3. 2.5–5% | 3. – | 3. Substantially enhanced maize and oat growth and decreased metal in plant tissue | |||
| 4. Nanoscale Zero-valent iron particles (nZVI) | 4. – | 4. CrVI+ | 4. – | 4. Reduction of Cr + 6 to Cr + 3 97.5% | 4. – | |||
| 5. nZVI | 5. Cd-spiked soil | 5. Cd | 5. 0.01% (g/g) | 5. – | 5. Rice seeds <10% and leaves <20% | |||
| 6. Iron phosphate (vivianite) nanoparticles | 6. Contaminated soil | 6. Pb | 6. 0.61–3.0 mg/g soil as PO4−3 | 6. TCLP leachable 85–95% | 6. Bioaccessible fraction 31–47% | |||
| 7. Synthesized apatite nanoparticles | 7. Pb-laden soil from a shoot range | 7. Pb | 7. 2 mL solution to 1 g soil | 7. TCLP leachable ~56% | 7. – | |||
| L | Coarse zeolite (pH = 9.5), fine zeolite (pH = 8.6), flue gas desulfurization (FGD) (pH = 11.2), fly ash (pH = 7.6), and biosolids (pH = 7.8) | Loamy-skeletal, mixed, mesic typic udorthents soil (top 10 cm; <2 mm fraction) with elevated Be and Cd concentrations from coal strip mining (Ohio); pH = 3–4 | Study targeted vegetative growth of soil with elevated levels of Be and Cd, which inhibits seed germination and root uptake of Ca and Mg and also degrades some proteins and enzymes | FGD 10% | Post-35 days | Pre: – | Application of FGD at 10% by weight increased soil pH from 3.1 to 5.0 and from 4.2 to 7.0, enhancing germination and rate of shoot elongation of lettuce; however, biosolids significantly enhanced soil aggregate stability and saturated water-holding capacity | [36] |
| Be: FGD reduced Be levels | Post: FGD exhibited the best remediation result among all the amendment materials, promoting the shoot growth to 9.6 cm | |||||||
| Cd: Not presented | % Reduction: – | |||||||
| Al: 97% of 8 mg/L | ||||||||
| L | Water treatment sludges (WTS) (pH = 7.2 and 6.3), red muds (pH = 10.7 and 12.2), and red gypsum (pH = 7.1) | Agricultural grassland soils (top 20 cm) contaminated by 19th century Cu and As mining at Devon Great Consols Mine, Devon, UK; pH = 4.6 | As and Cu | Water treatment sludges (WTS-A) 2% (w/w) | Reduction in pore water | Pre: – | WTS was the most effective amendment in terms of enhancing plant and microbial growth, decreasing metal and As mobility, and diminishing bioaccessible As. Rye grass and lettuce grown in WTS had the largest shoot biomass | [124] |
| Cu: 44% of 998 μg/L | Post: Rye grass and lettuce grown in WTS had the largest shoot biomass | |||||||
| As: 75% of 1,795 μg/L | % Reduction: – | |||||||
| L | Granulated blast furnace slag (GBFS) | Typical soil found in the Ostrobothnia region of Northern Finland | As, Cd, Cr, Cu, Ni, Pb, vanadium (V), and Zn | MI: GBFS 0.15, FA 0.15, PMS 0.30, GLD 0.10, L 0.30 | Residual fraction (not bioavailable): | Results for the use of GBFS with pulp and paper residues seem encouraging regarding the replacement of commercial fertilizers. However, with the use of converter steel slag, elevated total concentrations of Cr and V were detected. However, 46.0% of the total concentration of V occurred in the easily reduced fraction indicating potential bioavailability | [186] | |
| GSS-Converter steel slag | MII: GSS 0.15, FA 0.15, PMS 0.30, GLD 0.10, L 0.30 | As (MII) 61.7% | ||||||
| FA-Fly ash (P&P) | Cr (MI) 84.6% | |||||||
| GLD-Green liquor dregs (P&P) | According to the liming effect value of commercial ground limestone, which is 38% (Ca-equivalents, d.w.), 1.03 and 1.06 tons of Matrices I and II respectively would be needed to replace 1 ton of limestone | Ni (MI or MII) 53% | ||||||
| PMS-Paper mill sludge | Pb (MII) 90.3% | |||||||
| L-Lime waste | Cu (MI) 84.6% | |||||||
| L | Nano-Fe/CaO | Mica/fibrolite soils (<2 mm fraction) from Okayama prefecture, Japan | As, Cd, Cr, and Pb | Soil: Nano ratio | Decrease in metal surface amounts on soil surface | Not presented | The addition of nano-Fe/Ca/CaO immobilized 95–99% of heavy metals, versus 65–80% by simple grinding. Nano-Fe/Ca/CaO treatment reduced the concentration of leachates heavy metals to values lower than the Japan soil elution standard regulatory threshold of 0.01 mg/L for As, Cd, and Pb; and 0.05 mg/L for Cr | [187] |
| Nano-Fe/Ca/CaO | Soil: Nano-Fe/CaO (2/5) 10:1 | Cr 99% | ||||||
| Nano-Fe/Ca/CaO/PO4 | Soil:Nano-Fe/Ca/CaO (2/2/5) 10:1 | As 100% | ||||||
| Soil:Nano-Fe/Ca/CaO/PO4 (10:0.5:0.5 (NaH2PO4)) | Cd 99.4% | |||||||
| Pb 99.7% | ||||||||
| L/F | Steel shot (pH = 8.5), beringite (pH = 11), and municipal compost | Fine-grained spoil (top 0.3 m) of the former gold mine of Jales, Portugal; pH = 4.1 | As, Pb, Cd, and Zn | CBSS: Compost (5%) + Steelshot (1%) + Beringite (5%) | Post–443 days | When compared with the results of 1998, Holcus lanatus shoot growth in amended spoils increased 4–6 fold, depending on amendments. CBSS amendments decreased As plant uptake. Similar results were obtained for Cd and Zn | Over a 443-day period following spoil treatment, CBSS treatment decreased leached Cd, Zn, and Pb amounts and achieved the lowest increase in As leaching. Short-term plant experiments showed a better restoration of the vegetation cover from CBSS than compost alone | [109] |
| Leachate reduction: | ||||||||
| As: 661% | ||||||||
| Pb: 15% | ||||||||
| Zn: 0.2% | ||||||||
| Cd: 0.08% | ||||||||
| L | P-spiked Linz–Donawitz (LD) slag | Sandy Cu-contaminated soil (<2 mm fraction) from a former wood preservation site; pH = 5.7 | Cu | 0% (T1), 1% (T2), 2% (T3), and 4% (T4) per air-dried soil weight | Not presented | Post-2 weeks | All incorporation rates of LD slag increased the root and shoot dry weight yields compared to the untreated soil. Foliar Cu, Zn, and Cr concentrations of beans decreased with the LD slag incorporation rate | [188] |
| T2 highest bean growth and foliar Ca concentration, foliar Cu reduction below upper critical value, prevent excessive soil electrical conductivity and Zn deficiency | ||||||||
| L | Cyclonic ashes (Beringite) (pH = 9.5) | Soils in the southern Netherlands contaminated from Zn smelter emissions; pH = 5.2–5.8 | Cd and Zn | Cyclonic ashes (CA) 25% | Zn reduction in solution <100% of 9 μmol/L | Not presented | Initially, Zn decreases in all treatments, but only the CA treatment shows a continuous decrease to almost zero | [189] |
| Synthetic zeolites (pH = 10.1) | ||||||||
| L | Olive-mill compost (pH = 8.8) and fresh pig slurry (pH = 7.8) | Loamy sand mine spoil soil (top 10 cm; <2 mm fraction) from the mining area of La Unión-Cartagena, Spain; pH = 3.5 | As, Pb, and Zn | First application: | Reduction in pore water: | Pre: – | Addition of compost and pig slurry in combination with hydrated lime reduced the high metal mobility in soil, increased soluble nutrients concentrations, and allowed the growth of rye grass (Lolium perenne) in the soil. However, plant metal uptake increased | [190] |
| Compost 60 t/ha | 5-cm depth | Pb post – 1st harvest: 500 mg/kg | ||||||
| Pb: 94% of 2.67 mg/L | Pb post – 2nd harvest: 1,500 mg/kg | |||||||
| Second application (2 weeks later, combined with 15.5 t/ha of hydrated lime): | Zn: 85% of 3,296 mg/L | % Reduction: 67% | ||||||
| Compost 30 t/ha | ||||||||
| 12-cm depth | ||||||||
| Pb: 40% of 1.98 mg/L | ||||||||
| Zn: 62% of 3,100 mg/L | ||||||||
| F | Biosolids compost (pH = 6.9), leonardite (pH = 6.08), and sugar beet lime (SBL) (pH = 0.04) | Topsoil (top 15 cm; <2 mm fraction) contaminated by mine tailing spillage along the Guadiamar River (Spain); pH = 3.52 | Cu | Leonardite (LE) | Complexing capacity (CC) of humic acids from waste by-products (mol/kg) | Not presented | Biosolids slightly more effective in reducing bioavailable Cu(II); Cu precipitated as a hydroxide with pH increase | [191] |
| SBL | Pre: – | |||||||
| Unamended soil (S) | Post (LES2) 1.44 ± 0.04 | |||||||
| %Reduction: – | ||||||||
| BS 30 t/ha/y | ||||||||
| BIS2 − Soil + BS (2 years) | ||||||||
| BIS4 − Soil + BS (4 years) | ||||||||
| Soil + LE 25 t/ha/y | ||||||||
| SBL 10 t/ha/y | ||||||||
| LES2 − Soil + LE + SBL (2 years) | ||||||||
| LES4 − Soil + LE + SBL (4 years) | ||||||||
| L | Sepiolite | Soil (top 20 cm, <4 mm fraction) from agricultural fields in Jilin, China. The soils were artificially polluted with Cd (CdCO3): 1.25, 2.5, and 5 mg/k); pH = 7.76 | Cd, Zn | Sepiolite (S) applied to soils at 0, 0.5, 1, 3, and 5% | Post: – | Cd uptake by spinach | Sepiolite addition not only decreased Cd absorption in spinach and increased safety for edible vegetables, but it also improved the quality of Cd-contaminated soil. Results showed that the sepiolite treatments significantly increased soil pH, resulting in a decrease in the available form of Cd in soil. Concentrations in the edible parts of spinach at 1% sepiolite treatment were lower than MPC for vegetables at 1.25 mg/kg Cd concentration and met MPC at 5% sepiolite treatment under higher Cd concentrations of 2.5 and 5 mg/kg | [192] |
| Extractable Cd decrease: | 28–63.7% from 1.25 mg/kg | |||||||
| 9.8–44.6% from 1.25 mg/kg | 29.4–67.8% from 2.5 mg/kg | |||||||
| 14–33.5% from 2.5 mg/kg | 17.2–72.1% from 5 mg/kg | |||||||
| 15.1–22.5% from 5 mg/kg | ||||||||
| Apatite (pH = 7.8) and slovakite (pH = 8.7) | Contaminated loamy soil (top 30 cm, <2 mm fraction) from an old lead and zinc smelter site in Arnoldstein, Austria; pH = 6 | Pb, Zn, Cu, and Cd | Slovakite 5% (w/w) (S5) | Post-1 month | Phytoavailability reduction (DTPA extraction) | Apatite and slovakite amendments were both successful in lowering the potential bioavailability of metals, slovakite being more effective at the same weight share with the soil | [43] | |
| Apatite 5% (W/W) (A5) | Apatite (5%) Switch from carbonate and Fe and Mn oxide-bound fractions to less mobile and available chemical forms bound to soil organic component by factors of Pb 1.6, Zn 1.5, and Cd 3.4 | Pb (S5) 54% | ||||||
| Zn (S5) 55% | ||||||||
| Cu (S5) 19% | ||||||||
| Cd (A5) 52% | ||||||||
| L | Tourmaline | Soil (top 20 cm (<2 mm fraction) from the bank of the Dagu Drainage River in Tianjin, northern China; pH = 7.45 | Cd, Cu, Pb, and Zn | 5% (w/w) | Post-3 months | Post-3 months | Results indicated that tourmaline could decrease the available heavy metal content and transform heavy metals into less toxic forms. Tourmaline increased the maximum dry matter and total chlorophyll content of lettuce and decreased the Cd and Cu content in lettuce shoots | [193] |
| DTPA-extractable reduction | Lettuce plant growth 109.11% | |||||||
| Cd 20.41% from 0.49 ± 0.01 mg/kg | Chlorophyll 31.76% | |||||||
| Cu 19.20% from 8.02 ± 0.43 mg/kg | Lettuce shoots | |||||||
| Cd reduction 49.01% | ||||||||
| Cu reduction 30.90% | ||||||||
| F | Individual and mixed applications of fly ash, spent mushroom substrate, silkworm excrement, and limestone mine waste (pH ranged from 6.86 to 9.62) | Topsoil (<20 cm) contaminated by pyrite mine tailing spillage (China); pH = 5.62 | Cd | Fly ash (FA): 5% | FA 5% (Cd): 39.9% reduction reported | % reduction in bioavailable Cd: | Decreased Cd mobility, bioavailability, and leachability. Cd immobilized as carbonates, Fe–Mn oxides, and fraction bound with OM. Mixed amendment applications more effective than individual amendments; when applied alone, limestone and fly ash were most effective | [193] |
| Root (FA) 35.5% | ||||||||
| Shoot (FA) 46.9% | ||||||||
| Bean (FA) 46.6% | ||||||||
| Limestone, rock phosphate, palygorskite, and Ca Mg phosphate (CMP) | Calcareous urban soil (pH = 8.22) | Pb, Cd, Cu, and Zn | 2% phosphate (RP), palygorskite (PG), and CMP | Reductions in metal leaching | Not presented | Palygorskite and CMP were generally quite efficient in reducing the availability of metal, while rock phosphate was only efficient in acidic soil. The effects of rock phosphate in reducing metal availability in the calcareous soil were the least efficient | [194] | |
| Acidic urban soil (pH = 4.63) | Calcareous soil | |||||||
| Cd (CMP) 75.93% | ||||||||
| Cu (PG) 60.64% | ||||||||
| Pb (CMP) 74.12% | ||||||||
| Zn (PG) 64.38% | ||||||||
| Acidic soil | ||||||||
| Cd (RP) 79.06% | ||||||||
| Cu (PG) 49.64% | ||||||||
| Pb (RP) 69.44% | ||||||||
| Zn (PG) 43.77% | ||||||||
| F | Limestone (pH = 9.13), sepiolite (pH = 5.54), hydroxyapatite (pH = 8.21), and zeolite (pH = 10.61) | Soil (top 20 cm) contaminated from mining and smelting in the Shuikoushan Mine Zone in Hengyang City, southern Hunan Province, China; pH = 5.41 | Pb, Cd, Cu, and Zn | Combined amendments: Limestone + sepiolite (LS) | Post-4 months | Concentrations of Pb, Cd, Cu, and Zn in brown rice were decreased by 10.6–31.8%,16.7–25.5%, 11.5–22.1%, and 11.7–16.3%, respectively, as a result of 0.2–0.8% addition of LS, and decreased by 5.1–40.8%, 16.7–20.0%, 8.1–16.2%, and 13.3–21.7%, respectively, as a result of 0.2–0.8% addition of HZ | [195] | |
| Hydroxyapatite + zeolite (HZ) | The rice cultivar “II You93” | |||||||
| 0.2, 0.4, and 0.8% (w/w) |
5.6. Regulations
Regulatory frameworks and considerations for the beneficial use of waste materials set out to protect human health and the environment without creating major impediments that discourage beneficial use activities.[169] No encompassing U.S. federal program specifically addresses beneficial use activities across all industrial and commercial sectors. Federal regulations that do address the beneficial use of some waste materials include RCRA (40 CFR Part 261) and the Biosolids Rule (40 CFR Part 503). Additionally, many states have also developed regulatory programs that allow for the beneficial use of designated materials for specific applications. A list of state beneficial use programs is provided on EPA’s Industrial Materials Recycling Program website, http://www.epa.gov/wastes/conserve/imr/live.htm. These regulations operate under the common theme that the waste by-product must not be listed as a hazardous waste per state and federal regulations, must act as a suitable replacement for virgin or synthetic materials, and must not pose unacceptable risk to human health. These criteria help dictate the appropriate and safe use of a waste by-product, and, in the context of remediation, typically require permanent monitoring of the soil condition and groundwater quality. Besides the federal and state-level frameworks, additional regulations should be considered when deciding whether to use a waste by-product in place of a traditional amendment (Table 6). Additionally, if using waste by-products for beneficial use outside of the U.S., national and local standards and limits must also be considered. Still, unintended consequences can occur in environmental work. Solutions to one environmental problem may lead to another and environmental scientists, engineers, and geologists must carefully evaluate all potential positive and negative impacts before implementing a remediation program.
Table 6.
Regulatory considerations for selected amendments.
| Waste by-product | Description of the regulatory consideration |
|---|---|
| Biosolids | The Clean Water Act Biosolids Rule (40 CFR Part 503) requires sites to get a Class B permit (site restrictions). It may be possible to compost or otherwise treat the biosolids on site to reach Class A quality (with no site restrictions). For Comprehensive Environmental Response, Compensation, and Liability Act of 1980 (CERCLA) actions, no permit is required, but sites should adhere to the spirit of state and local permit requirements (applicable or relevant and appropriate requirements [ARARs]) when possible. State-specific regulations also may apply |
| State solid waste permits may be required to land apply biosolids | |
| Manures | Federal and state best management practice (BMP) nutrient management; concentrated animal feeding operations may have bookkeeping requirements |
| Pulp sludges | Dioxin concentrations are restricted—voluntary or required by state standard 10 ppt toxic equivalent (TEQ) for dioxin incorporated. May have high Na, which can limit applications |
| Lime | State-specific lime-labeling requirements |
| Wood ash | May be regulated as a caustic material; pH will decrease to 8.3 with exposure to air; state-specific soil amendment or liming material regulations |
| Coal combustion residuals (CCRs) | State-specific regulations. Coal mining site regulation under Surface Mining Control and Reclamation Act of 1977 (SMCRA) is expected by 2008 |
| Red mud (bauxite) | Regulated as mining waste in situ, but labeled for application as a soil amendment by many states/localities |
| Foundry sand and steel slag | State-specific; different states may have restriction by grade |
| Cement kiln dust (CKD) | Standards for the management of CKD were proposed in 1999 (64 FR 45632), but a final rule has not been promulgated as of the publication date of this report |
| Dredged materials | U.S. Army Corps of Engineers (USACE) regulations (to pull out of waterway) as well as state-specific (to land apply) |
5.7. Potential unintended consequences associated with waste by-products
Although immobilization techniques using waste by-products and other natural and synthetic amendments can effectively immobilize metals and support a healthy subsurface environment, there are unintended consequences and potential limitations that must be considered so as to not increase risks to human health and the environment.[196,197] Not all unintended consequences will be fully understood or predicted prior to implementing a treatment option, but they may generally fall under the categories discussed below.
5.7.1. Interactions with amendment constituents
Interactions between amendment and soil geochemistry and target versus non-target metals can increase metal mobility and bioavailability. For example, Cd bioavailability is strongly affected by Zn at typical background concentrations in soil, as Zn inhibits Cd binding to soil, root uptake, and transport. In most aerobic soils, metals such as Zn and Cd are present as complexed cations or anions but, depending on soil conditions, constituents in amendments can interact with soil particles in unintended ways.[198] Iron, Al, and Mn oxide soil minerals act as important trace metal sinks in amended soils.[199-201] Trace metal sorption is a pH-dependent process where protons compete with cations for sorption. Thus, metal cation adsorption by oxide surfaces increases to almost 100% with increasing pH, whereas oxyanion adsorption generally decreases with increasing pH.[202] For example, laboratory study conducted by Aguilar-Carrillo[170] reported As, Cd, and Tl immobilization with PG and sugar foam (SF) treatment. In comparison to PG + SF treatments, the single SF amendment showed the highest retention of the three elements (Table 7).
Table 7.
Mean concentration values (n = 6; mM kg−1) and percentage with respect to the initial element concentration (in parenthesis) of As, Cd, and Ti that is retained in the soil columns after the incubation period.
| As | Cd | Tl | |
|---|---|---|---|
| C | 20.8 (70) | 12.6 (88) | 4.2 (60) |
| SF | 26.0 (87) | 14.2 (99) | 6.1 (87) |
| PG + SF | 25.0 (84) | 13.3 (93) | 5.2 (74) |
| PSE | 0.18 | 0.07 | 0.04 |
The addition of PG and SF induced the formation and retention of Al-hydroxypolymer in acidic soils, to which As, Cd, and Tl were associated, probably through direct coordination or the formation of ternary complexes.[170] Metal cations can also sorb to OM in soil with increased pH and reduce the corresponding solubility of the metals present in amended soils.[24] Metal cations may also form soluble precipitates with phosphate, sulfides, and other anions[198,203] based on increasing pH in most cases.
5.7.2. Long-term stability
Metals stability (e.g., fixation) can occur in a variety of ways, including solid phase diffusion into mineral lattices, mineral entrapment (e.g., between clay layers), and coprecipitation. If physicochemical properties change over time, metals can remobilize (e.g., desorb) in the subsurface environment. For example, OM is important for the retention of metals by soil solids, thus decreasing mobility and bioavailability. However, because of the complexation of metals by soluble OM, the addition of OM can release metals from the solids to the soil solution. The increase in the solubility of the Cu and Pb is related to the dissolution of the humic acid (HA) component of the OM, which indicates that solution phase speciation reactions with OM dominate the partition of these metals at higher pH because the dissolved organic matter (DOM) increases because of the solubility of HA under high pH. Elevated temperatures generally increase metal “aging,” whereby metals move into inaccessible porous mineral surfaces and form more stable bonds over time. If OM is present, higher temperatures can also increase microbial attenuation and desorb or remobilize the aged metals through OM degradation.[196,197] Therefore, the application methods and soil hydrogeochemistry are crucial in determining the long-term stability of treatment with waste by-products, as well as synthetic or natural amendments. For example, applying an amendment without mixing it into the soil homogeneously may decrease the likelihood of long-term success, as would applying the amendment at a lower application rate (e.g., due to uncertainty in the effectiveness rate). Similarly, changes in the soil hydrogeochemistry (e.g., decreased pH or reducing conditions) may remobilize metals. In certain cases, the amendment itself may cause a nutrient deficiency or excess fertility, or otherwise adversely affect soil structure, OM, or other conditions. [196,197]
5.7.3. Leaching of contaminants
Immobilized metals can remobilize under changing pH conditions as certain metals become more soluble at pH < 6.0,[89] while others become less soluble at higher pH. Indeed, it is apparent that alkaline amendments low in calcium (e.g., certain zeolites) and high in DOM promote leaching in the subsurface environment. Beesley et al.[147] found that the application of both Alperujo compost and biochar increased the potential for As leaching in a laboratory study designed to treat As-, Cd-, Cu-, Pb-, and Zn-contaminated soil (Fig. 4). Arsenic leaching was caused due to the influence of pH, degradable organic carbon (DOC), and soluble P concentrations as staggering factors on the geochemistry of trace elements.[147]
Figure 4.
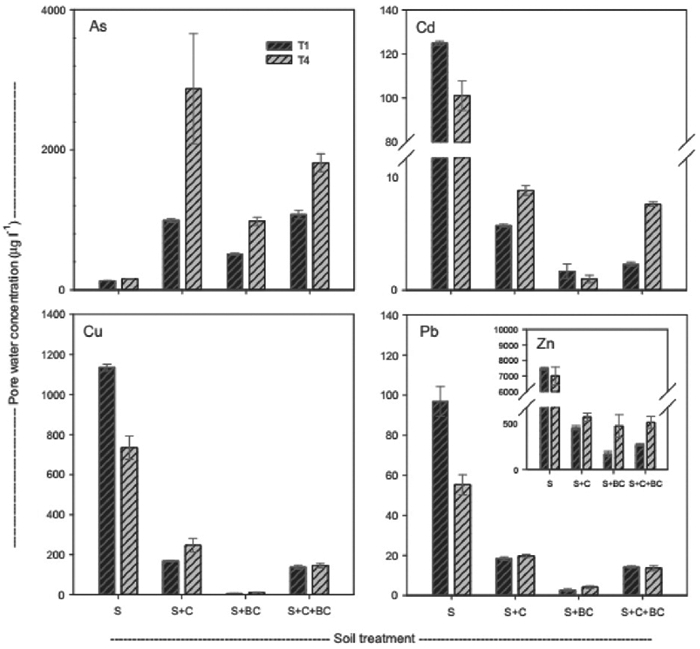
Concentration of arsenic (As) and selected heavy metals in pore water from soil (S), soil plus compost (S + C), soil plus biochar (S + BC), and soil plus compost and biochar (S + C + BC) sampled 1 week (T1) and 4 weeks (T4) following commencement of the experiment (mean n = 4; ±se).[147] © Elsevier. Reproduced by permission of Elsevier. Permission to reuse must be obtained from the rightsholder.
Carcamo et al.[56] conducted a field study that reduced Cu and Zn availability using seashell grit, but As still remained soluble in pore water. Gonzalez et al.[178] conducted a laboratory study designed to treat As-, Cd-, Cu-, Pb-, and Zn-contaminated soil with sludge, compost, or synthetic iron oxides. Study findings indicated that amendments that raised pH, especially marble sludge, effectively diminished soluble heavy metal concentrations. However, these amendments also increased As concentration in lixiviates and encouraged As dispersion. Iron oxides that fixed As were not effective in diminishing soluble heavy-metal concentrations at acidic pH. Houben et al.[204] conducted a laboratory study that aimed to reduce Cd, Pb, and Zn concentrations in soil using bone meal, manure, or iron grit. The study found that Pb leaching was strongly affected by DOC release. Therefore, bone meal and manure treatments, which highly increased DOC concentrations in leachates, increased the flow-weighted mean Pb concentrations by 2.3 and 16 times, respectively. From these studies, it is apparent that amendments can beneficially stabilize metal-contaminated soil, but the potential for leaching of one or more metal contaminants is also present under certain conditions given certain subsurface conditions and amendment physicochemical characteristics. Establishment of field trials on metal-contaminated soils and sediments should be conducted prior to further assessment.
5.7.4. Inconsistency of the waste by-product composition
Using a waste by-product as an amendment for in situ remediation may be challenging if the supply fluctuates over time, seasons, etc. For example, if a more economically valuable alternative arises that creates an additional market for a given waste by-product (e.g., ethanol), or if an industry reduces its waste footprint voluntarily or due to new regulations (e.g., food waste), it may no longer be feasible to use a specific waste by-product or by-product class for remediation. In certain cases, more likely than not the composition of waste by-products can be inconsistent due to spatial (e.g., different facilities) or temporal (e.g., different inputs over time) changes in the waste. For example, biosolids and boiler ash can vary in their physicochemical properties based on changing inputs, variation among facilities, and even intrafacility variation.[205] These changes could potentially lead to performance issues if in situ remediation of metal-contaminated soil and sediment is conducted under the assumption that a waste by-product amendment will include a certain physicochemical profile.
5.7.5. Introduction of additional or new contaminants
Depending on which metals are present, and their concentration and physiochemical characteristics, cumulative additions of amendments may result in increasing metal content over time with potential adverse effects on the subsurface environment. Consequently, using waste by-products may introduce additional mass of metal contaminants already present in soil, or potentially even new metal constituents into the contaminated medium.[196,197] Soil structure can be damaged by constituents in certain amendments (e.g., Na in zeolite) and soil porosity may be reduced (e.g., steel shot).[94] Lee et al.[184] conducted a laboratory study using one of the following amendments: bone meal, bottom ash, furnace slag, red mud, or plant species to reduce Cd, Cu, Pb, and Zn concentrations in soil. The study found that bone meal increased the concentration of all metals evaluated in pore water samples, though the study was unable to identify whether it was related to elevated metal concentrations in the bone meal itself or the effect the amendment had on the soil hydrogeochemistry.
5.7.6. Plant uptake
Soil chemical processes typically limit the availability of metals for uptake by plants. However, during in situ remediation of contaminated metals, it is possible for plant uptake (e.g., similar to phytoremediation) to occur rather than, or in concert with in situ immobilization/stabilization of metals, depending on site conditions such as the vertical extent of contamination and the natural ground cover at the site. Houben et al.[204] conducted a laboratory study that aimed to reduce Cd, Pb, and Zn concentrations in soil using bone meal, manure, or iron grit. Iron grit induced strong Cd and Pb leaching reductions and doubled Cd and Pb concentrations in shoots of white lupin. Lee et al.[184] conducted a laboratory study aiming to reduce Cd, Pb, and Zn concentrations in soil using zero-valent iron, limestone, sludge, bone meal, or bottom ash. The study found that bottom ash increased Cd concentrations in lettuce shoots compared to untreated soil. Another laboratory study designed to reduce As, Pb, and Zn concentrations in soil using olive mill compost or fresh pig slurry found that plant metal uptake increased, although the metal mobility in soil was reduced.[190] Nevertheless, even in cases where plant uptake may occur, senescence due to phytotoxicity often decreases the likelihood that plants with high levels of metal contamination could be ingested by ecological or human receptors. Metal phytotoxicity (e.g., Al, As, B, Cr3+, Cu, fluorine [F], Mn, Ni, or Zn) can limit the overall metal concentration in plant shoots to levels that are typically tolerated by ecological and human receptors, meaning the plant would likely die long before it can accumulate enough metal to harm humans through consumption. Additionally, metal adsorption or precipitation in soil and roots usually limits the amount of uptake to plant shoots, and the presence of certain metals in conjunction with one another can reduce overall toxicity.[196,197] For example, the presence of Zn significantly reduces Cd absorption by animals.[206] Another study found that increased Zn levels in spinach and lettuce reduced the absorption of Cd in leafy vegetables consumed by Japanese quail.[207] Increased dietary Zn has also been shown to strongly inhibit Cd absorption in cattle.[208] Plant uptake of Cd and Zn can also be reduced by liming the soil to increase pH.[196]
Unfortunately, certain metals are not restricted by the soil-plant barrier (e.g., Se, Mo, Cd, Co) to protect against the ingestion of plants by human or ecological receptors. For example, earthworms still exhibit similar metal bioaccumulation even with liming of soil to increase pH, showing that reduced metal concentrations in pore water cannot be used to estimate exposure for soil ingested by invertebrates.[196] Consequently, it is crucial to characterize potential risks at a site and, as necessary, enlist engineering (e.g., paving, fencing) or administrative (e.g., restrictive use covenants) controls in locations with the potential for ecological or human exposures to contaminated soil or plants.
5.8. Results comparison to background and benchmark values
Determining unacceptable risks to human health and the environment is often difficult from a decision-making perspective. One major reason why waste by-products are not beneficially used to their full capacity in the United States is an overall lack of data and general uncertainty regarding the risks, benefits, and unintended consequences of using waste by-products.[4] In an attempt to address this uncertainty, we have compiled metal concentrations presented in the literature in order to compare them against the concentrations in the 2013 U.S. Geological Survey (USGS) report[4], which analyzed background metal concentrations in surface soil throughout the United States (Table S5). Two different extraction methods were used for As and Cd, but, for summary purposes, the minimum and maximum concentrations were incorporated in one column. From the studies reviewed for this report, concentration data were available for 18 metals that were contained in waste by-products. The concentrations of eight of these metals (Ag, As, Cd, Cu, Cs, Mo, Pb, and Zn) exceed the median U.S. background soil concentration for at least one waste by-product sample out of the three waste by-product categories. Additionally, three metals (Mn, Se, and V) exceed the maximum U.S. background soil concentration for at least one organic waste by-product sample. Overall, the organic waste by-products contained a greater proportion of elevated metals concentrations compared to U.S. background soils, which are based on samples collected from 0–100 cm below the surface. Finally, seven metals (Ba, Be, Co, Cr, Hg, Ni, and Sb) did not exceed median or maximum U.S. background soil concentrations for any waste by-product samples. These data indicate that there could be some potential concern for elevated metal concentrations in certain waste by-product samples. Variability among the waste by-product categories may also exist. However, the question as to whether metal concentrations above median or maximum U.S. background concentrations pose a potential risk to humans or ecological receptors still exists.
To demonstrate whether metals exceeding background concentration in waste by-products could pose a potential human health or environmental risk, Table S6 compares human and ecological soil screening values. Screening values were reviewed for 11 metals; however, Ag and Cs do not have benchmark values, so they were removed from the comparison. Overall, three metals (As, Mn, and V) exceeded the human soil screening level (SSL) based on the maximum metal concentration identified in the waste by-product studies reviewed for the report. Additionally, eight (As, Cd, Cu, Mn, Pb, Se, V, and Zn) metals exceeded the eco-SSL based on the maximum metal concentration identified in the waste by-product studies reviewed for the report. The eco-SSLs tend to contain more conservative values to account for the likelihood that ecological species living in or near the soil will be more greatly exposed to metal contaminants. It should be noted, however, that these values are only considered for preliminary risk screening and do not take into account site-specific conditions, site-specific risk assessment modeling, and the unique conditions that can be present at contaminated sites with pending remediation.
If waste by-products are being considered as amendments for the in situ remediation of contaminated soil and sediment, the potential for adding elevated metals concentrations from the waste by-product itself may not be a limiting factor if (1) the metal may also become reduced or immobilized during the in situ treatment, (2) the metal concentration and toxicity may be considered minor compared to the contaminant present at the site, and/or (3) there may not be human or ecological receptors likely to be exposed to the added metal concentrations at the site (e.g., for industrial or vacant sites in non-sensitive ecological areas).
6. Summary and recommendations
Selected waste by-products have been shown to be successfully used as amendments for the in situ remediation of soil and sediment. This review presents the current state of science of using waste by-products as amendments for in situ remediation of metal-contaminated soil and sediment. Moreover, it also summarizes the multimedia, probabilistic human health and environmental risk assessment that compares the waste concentrations to the actual likelihood of exposure, the corresponding toxicity for each metal, and the resulting risk that could be used to provide a more definitive understanding of the “appropriateness” of using waste by-products from a human health and environmental perspective.
To better estimate human health and environmental risk and the suitability of amendment substitution, additional physicochemical properties and composition data for waste by-products are needed. Further investigation of the chemical and physical properties and metals reduction data for specific and promising amendments would be worthwhile to better identify waste by-products as substitute amendments. Such an investigation could focus on comparing amendments within the organic, liming, and mineral categories first, and then comparing property data across the categories. A targeted list of properties that are important for metal immobilization mechanisms, and could be the focus of further investigations of amendments, include pH, OC, surface area, Eh, CEC, adsorption capacities, Fe and Mn oxides, and carbonate content. As additional properties data become available, it could be stored by amendment or amendment combinations in a web-based, searchable tool or searchable access database to facilitate queries and data analyses. The addition of the annual generation quantities, availability, source location, and costs related to the purchase and transportation of the waste by-products will provide supplementary decision-making information for site managers, regulators, and communities. Additionally, longer-term laboratory and field studies are needed that consider amendment aging and the interactions between metal contaminants in post-treatment soil with the application of amendments that may also contain elevated metal concentrations.
Supplementary Material
References
- U.S. EPA. (2014, 30 June). Land risk management research, contaminated soil and sediments. Retrieved 14 July, from http://www.epa.gov/nrmrl/lrpcd/contamin_ss.html [Google Scholar]
- U.S. EPA. (2009). Technology performance review: Selecting and using solidification/stabilization treatment for site remediation. (EPA/600/R-09/148). Washington, DC: US EPA. [Google Scholar]
- Rajendram P, Muthukrishnan J, and Gunasekaran P (2003). Microbes in heavy metal remediation. Indian J. Exp. Biol, 41, 935–944. [PubMed], [PubMed] [Google Scholar]
- Smith DB, Cannon WF, Woodruff LG, Solano F, Kilburn JE, and Fey DL (2013). Geochemical and mineralogical data for soils of the conterminous United States. Chapter 2, Tables 2 and 3. U.S. Geological Survey Data Series 801 Retrieved from http://pubs.usgs.gov/ds/801/ [Google Scholar]
- U.S. EPA. (2007b). Treatment technologies for mercury in soil, waste, and water. (EPA-542-R-07-003). Washington, DC: US EPA; Retrieved from http://www.epa.gov/tio/download/remed/542r07003.pdf [Google Scholar]
- McLaughlin MJ, Hamon RE, McLaren RG, Speir TW, and Rogers SL (2000a). Review: A bioavailability-based rationale for controlling metal and metalloid contamination of agricultural land in Australia and New Zealand. Aust. J. Soil Res, 38, 1037–1086. [Google Scholar]
- McLaughlin MJ, Zarcinas BA, Stevens DP, and Cook N (2000b). Soil testing for heavy metals. Commun. Soil Sci. Plan, 31, 1661–1700. [Google Scholar]
- Wuana RA, and Okieimen FE (2011). Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecology. [Google Scholar]
- Evanko CR, and Dzombak DA (1997). Remediation of metals-contaminated soils and groundwater. Carnegie Mellon University: GWRTAC (Ground-Water Remediation Technologies Analysis Center). Report No.: Technology Evaluation Report TE-97-01. [Google Scholar]
- Mulligan CN, Yong RN, and Gibbs BF (2001). An evaluation of technologies for the heavy metal remediation of dredged sediments. J. Hazard. Mater, 85, 145–163. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. (2007). The use of soil amendments for remediation, revitalization and reuse. Washington, DC: U.S. Environmental Protection Agency (U.S. EPA) Retrieved from http://www.clu-in.org/download/remed/epa-542-r-07-013.pdf [Google Scholar]
- U.S. EPA. (2005). Chapter 5.0 In Situ Capping. The contaminated sediment remediation guidance for hazardous waste sites. (EPA-540-R-05-012). Washington, DC: US EPA; Retrieved from http://www.epa.gov/tio/download/remed/542r07003.pdf [Google Scholar]
- U.S. EPA. (2013d). Use of amendments for in situ remediation at superfund sediment sites. (OSWER Directive 9200.2-128FS). Washington, DC: US EPA; Retrieved from http://www.epa.gov/superfund/health/conmedia/sediment/pdfs/In_situ_AmendmentReportandAppendix_FinalApril2013.pdf [Google Scholar]
- Jardine PM, Parker JC, Stewart MS, Barnett MO, and Fendorf SE (2007). Decreasing toxic metal bioavailability with novel soil amendment strategies: This report was prepared under contract to the Department of Defense Strategic Environmental Research and Development Program (SERDP). [Google Scholar]
- U.S. EPA. (1995). Contaminants and remedial options at selected metals-contaminated sites. (EPA/540/R-95/512). Washington, DC: Retrieved from http://www.epa.gov/superfund/health/conmedia/sediment/pdfs/ch5.pdf [Google Scholar]
- U.S. EPA. (2007c). Chapter 3: Environmental chemistry, transport and fate framework for metals risk assessment. Washington, DC: US EPA. [Google Scholar]
- Martin F, Garcia I, Dorronsoro C, Simon M, Aguilar J, Ortiz I, Fernandez J (2004). Thallium behavior in soils polluted by pyrite tailings (Aznalcollar, Spain). Soil Sediment Contam., 13, 25–36. [Google Scholar]
- Violante A, Cozzolino V, Perelomov L, Caporale AG, and Pigna M (2010). Mobility and bioavailability of heavy metals and metalloids in soil environments. J. Soil Sci. Plan. Nutr, 10, 268–292. [Google Scholar]
- McLean JE, and Bledsoe BE (1992). Ground water issue: Behavior of metals in soils. Superfund Technology Support Center for Ground Water, Technology Innovation Office, Office of Solid Waste and Emergency Response, Office of Research and Development. Report No.: EPA/540/S-92/018. [Google Scholar]
- Shuman LM (1991). Chemical forms of micronutrients in soils In McLean JE and Bledsoe BE (Eds.), Ground water issue: Behavior of metals in soils: Superfund technology support center for ground water. Technology Innovation Office, Office of Solid Waste and Emergency Response, Office of Research and Development, EPA/540/S-92/018. [Google Scholar]
- Sposito G (1984). The surface chemistry of soils. Oxford, England: Oxfod Univ. Press. [Google Scholar]
- Bolan NS, Adriano DC, Mani A, and Duraisamy A (2003). Immobilization and phytoavailability of cadmium in variable charge soils. II. Effect of lime addition. Plant Soil, 251, 187–198. [Google Scholar]
- Choppala G, Bolan N, Kunhikrishnan A, Skinner W, and Seshadri B (2015). Concomitant reduction and immobilization of chromium in relation to its bioavailability in soils. Environ. Sci. Pollut. R, 22, 8969–8978. [DOI] [PubMed] [Google Scholar]
- Adriano DC (2001). Trace elements in the terrestrial environments: Biogeochemistry, bioavailability, and risks of heavy metals (2nd ed.). New York: Springer-Verlag. [Google Scholar]
- Harter RD, and Naidu R. (1995). Role of metal-organic complexation in metal sorption by soils. Adv. Agron, 55, 219–263. [Google Scholar]
- Haas CN, and Horowitz ND (1986). Adsorption of cadmium to kaolinite in the presence of organic material. Water Air Soil Poll., 27, 131–140. [Google Scholar]
- U.S. EPA. (1992). Framework for ecological risk assessment Risk Assessment Forum. (EPA/505/2-90/001). Washington, DC: US EPA. [Google Scholar]
- Borda MJ, and Sparks DL (2008). Mobility of trace elements in soil environments In: Violante A, Huang PM, and Gadd GM (Eds.), Biophysico-chemical processes of metals and metalloids in soil environments (pp. 97–168), Hoboken, NJ: John Wiley and Sons. [Google Scholar]
- Plant Conservation Alliance (PCA). (2002). Using biosolids for reclamation and remediation of disturbed soils: Prepared by the Center for Urban Horticulture, University of Washington for the Plant Conservation Alliance, Bureau of Land Management, United States Department of Interior. [Google Scholar]
- Scheckel KG, Diamond GL, Burgess MF, Klotzbach JM, Maddaloni M, Miller BW, and Serda SM (2013). Amending soils with phosphate as means to mitigate soil lead hazard: A critical review of the state of the science. J. Toxicol. Env. Health B, 16, 337–380. [DOI] [PubMed] [Google Scholar]
- Boisson J, Mench M, Vangronsveld J, Ruttens A, Kopponen P, and De Koe T (1999). Immobilization of trace metals and arsenic by different soil additives: Evaluation by means of chemical extractions. Commun. Soil Sci. Plan, 30, 365–387. [Google Scholar]
- Pearson MS, Maenpaa K, Pierzynski GM, and Lydy MJ (2000). Effects of soil amendments on the bioavailability of lead, zinc, and cadmium to earthworms. J. Environ. Qual, 29, 1611–1617. [Google Scholar]
- Bolan NS, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park JE, Makino T, Kirkham MB, Scheckel K (2014). Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater, 266, 141–166. [DOI] [PubMed] [Google Scholar]
- Gadepalle VP, Ouki SK, Van Herwijnen R, and Hutchings T (2007). Immobilization of heavy metals in soil using natural and waste materials for vegetation establishment on contaminated sites. Soil Sediment Contam., 16, 223–251. [Google Scholar]
- Barthel J, and Edwards S (2001). Chemical stabilization of heavy metals Metals Treatment Technologies (MT2), L.L.C. Wheat Ridge, CO: Retrieved from http://www.containment.fsu.edu/cd/content/pdf/040.pdf [Google Scholar]
- Liu R, and Zhao D (2013). Synthesis and characterization of a new class of stabilized apatite nanoparticles and applying the particles to in situ Pb immobilization in a fire-range soil. Chemosphere, 91, 594–601. [DOI] [PubMed] [Google Scholar]
- Liu RQ, and Zhao DY (2007). In situ immobilization of Cu(II) in soils using a new class of iron phosphate nanoparticles. Chemosphere, 68, 1867–1876. [DOI] [PubMed] [Google Scholar]
- An B, and Zhao DY (2012). Immobilization of As(III) in soil and groundwater using a new class of polysaccharide stabilized Fe-Mn oxide nanoparticles. J. Hazard. Mater, 211, 332–341. [DOI] [PubMed] [Google Scholar]
- Clu-In. (2014c, February 20). Solidification: Overview. Table 1. Retrieved 24 Sept 2014, from http://clu-in.org/techfocus/default.focus/sec/Solidification/cat/Overview/ [Google Scholar]
- Abdel-Kader NRS, and K. H (2013). Assessment of heavy metals immobilization in artificially contaminated soils using some local amendments. Open J. Metal, 3, 8–76. [Google Scholar]
- Minocha AK, and Goyal MK (2013). Immobilization of molybdenum in ordinary Portland cement. J. Chem. Eng. Process Technol, 4, 162. [Google Scholar]
- Savannah River Ecology Laboratory. (2014). Soil remediation using in situ immobilization techniques. Retrieved July 28, 2014, from http://srel.uga.edu/outreach/snapshots/soil_remediation.html [Google Scholar]
- Tica D, Udovic M, and Lestan D (2011). Immobilization of potentially toxic metals using different soil amendments. Chemosphere, 85, 577–583. [DOI] [PubMed] [Google Scholar]
- Finžgar N, Kos B, and Leštan D (2006). Bioavailability and mobility of Pb after soil treatment with different remediation methods. Plant Soil Environ., 52, 25–34. [Google Scholar]
- Petrilakova A, and Balintova M (2011). Utilization of sorbents for heavy metals removal from acid mine drainage. Kosice, Slovakia: University of Kosice; Retrieved from http://www.nt.ntnu.no/users/skoge/prost/proceedings/pres2011-and-icheap10/PRES11/214Petrilakova.pdf [Google Scholar]
- Cao RX, Ma LQ, Chan M, Singh SP, and Harris WG (2003). Phosphate-induced metal immobilization in a contaminated site. Environ. Poll, 122, 19–28. [DOI] [PubMed] [Google Scholar]
- Chmielewska E, Hodossyova R, and Bujdos M (2013). Kinetic and thermodynamic studies for phosphate removal using natural adsorption materials. Pol. J. Environ. Stud, 22, 1307–1316. [Google Scholar]
- Brown L, Seaton K, Mohseni R, and Vasiliev A (2013). Immobilization of heavy metals on pillared montmorilonite with a grafted chelate ligand. J. Hazard Mater, 15, 181–187. [DOI] [PubMed] [Google Scholar]
- Cruz-Guzman M, Celis R, Hermosin MC, Koskinen WC, Nater EA, and Cornejo J (2006). Heavy metal adsorption by montmorillonites modified with natural organic matter. Soil Sci. Soc. Am. J, 70, 215–221. [Google Scholar]
- Addy MA (2011). Modified organoclay containing chelating ligand for adsorption of heavy metals in solution East Tennessee State University School of Graduate Studies Electronic Theses and Dissertations, Paper 1372. Retrieved from http://dc.etsu.edu/etd/1372 [Google Scholar]
- Hyman M, and Dupont RR (2001). Groundwater and soil remediation: Process design and cost estimating of proven technologies. ASCE Press. [Google Scholar]
- Warren GP, and Alloway BJ (2003). Reduction of arsenic uptake by lettuce with ferrous sulfate applied to contaminated soil. J. Environ. Qual, 32, 767–772. [DOI] [PubMed] [Google Scholar]
- Walker DJ, Clemente R, and Bernal MP (2004). Contrasting effects of manure and compost on soil pH, heavy metal availability and growth of Chenopodium album L. in a soil contaminated by pyritic mine waste. Chemosphere, 57, 215–224. [DOI] [PubMed] [Google Scholar]
- Asensio V, Covelo EF, and Kandeler E (2013). Soil management of copper mine tailing soils—Sludge amendment and tree vegetation could improve biological soil quality. Sci. Total Environ, 456–457. [PubMed], [DOI] [PubMed] [Google Scholar]
- Brown S, Svendsen A, and Henry C (2009). Restoration of high zinc and lead tailings with municipal biosolids and lime: A Field Study. J. Environ. Qual, 38, 2189–2197. [DOI] [PubMed] [Google Scholar]
- Carcamo V, Bustamante E, Trangolao E, de la Fuente LM, Mench M, Neaman A, and Ginocchio R (2012). Simultaneous immobilization of metals and arsenic in acidic polluted soils near a copper smelter in central Chile. Environ. Sci. Poll. R, 19, 1131–1143. [DOI] [PubMed] [Google Scholar]
- Ginocchio R, Cárcamo V, Bustamante E, Trangolao E, de la Fuente LM, and Neaman A (2013). Efficacy of fresh and air-dried biosolids as amendments for remediation of acidic and metal-polluted soils: A short-term laboratory assay. J. Soil Sci. Plant Nutr, 13, 855–869. [Google Scholar]
- Rate AW, Lee KM, and French PA (2004). Application of biosolids in mineral sands rehabilitation: Use of stockpiled topsoil decrease trace elements by plants. Biores. Technol, 3, 223–231. [DOI] [PubMed] [Google Scholar]
- Soler-Rovira P, Madejon E, Madejon P, and Plaza C (2010). In situ remediation of metal-contaminated soils with organic amendments: Role of humic acids in copper bioavailability. Chemosphere, 79, 844–849. [DOI] [PubMed] [Google Scholar]
- Carrillo Zenteno MD, de Freitas RCA, Fernandes RBA, Fontes MPF, and Jordao CP (2013). Sorption of cadmium in some soil amendments for in situ recovery of contaminated soils. Water Air Soil Poll., 224, 1418. [Google Scholar]
- Venner KH, Prescott CE, and Preston CM (2009). Leaching of nitrogen and phenolics from wood waste and co-composts used for road rehabilitation. J. Environ. Qual, 38, 281–290. [DOI] [PubMed] [Google Scholar]
- Zeng M, Campbell AG, and Mahler RL (1993). Log yard fines as a soil amendment: 601 pot and field studies. Commun. Soil Sci. Plant Anal, 24, 2025–2041. [Google Scholar]
- Preston CM, and Forrester PD (2004). Chemical and carbon-13 cross-polarization 567 magic-angle spinning nuclear magnetic resonance characterization of logyard fines 568 from British Columbia. J. Environ. Qual, 33, 767–777. [DOI] [PubMed] [Google Scholar]
- Jirjis R, and Theander O (1990). The effect of seasonal storage on the chemical 495 composition of forest residue chips. Scand. J. Res, 5, 437–448. [Google Scholar]
- Petruzzelli G, Barbafieri M, Bretzel F, and Pezzarossa B (1998). In situ attenuation of heavy metal mobility in contaminated soil by use of paper mill sludge Contaminated Soil (pp. 1139–1140). London: Thomas Telford Publishing. [Google Scholar]
- McBride MB (1994). Environmental chemistry of soils. New York: Oxford University Press. [Google Scholar]
- U.S. EPA. (2013a). Coal combustion residues. Wastes—non-hazardous waste—industrial waste. Retrieved September 2014, from http://www.epa.gov/osw/nonhaz/industrial/special/fossil/coalashletter.htm [Google Scholar]
- U.S. EPA. (2013b). Frequent questions: Coal combustion residues (CCR)—Proposed rule. Wastes—non-hazardous waste—industrial waste. Retrieved September 2014, from http://www.epa.gov/waste/nonhaz/industrial/special/fossil/ccr-rule/ccrfaq.htm [Google Scholar]
- Chang YT, Hsi HC, Hseu ZY, and Jheng SL (2013). Chemical stabilization of cadmium in acidic soil using alkaline agronomic and industrial by-products. J. Environ. Sci. Heal. A, 48, 1748–1756. [DOI] [PubMed] [Google Scholar]
- Gangloff WJ, Ghodrati M, Sims JT, and Vasilas BL (2000). Impact (ed.) The reuse and recycling of contaminated soil. Lewis Publ., of fly ash amendment and incorporation method on hydraulic properties of a sandy soil. Water Air Soil Poll., 119, 231–245. [Google Scholar]
- Bern J (1976). Residues from power generation: Processing, recycling, and disposal in land application of waste materials. Soil Conservation Society of America, 226–248. [Google Scholar]
- Page AL, Elseewi AA, and Straughan IR (1979). The physical and chemical properties of fly ash from coal-fired power plants with soils Prentice Hall, Upper Saddle River, NJ: reference to environmental impacts; Residue Rev., 71, 83–120. [Google Scholar]
- Lau SSS, Fang M, Wong JWC (2001). Effects of composting process and fly ash amendment on phytotoxicity of sewage sludge. Arch. Environ. Contam. Toxicol, 40, 184–191. [DOI] [PubMed] [Google Scholar]
- Zinck J, and Griffith W (2006). Utilizing industrial wastes and alternative reagents to treat acidic drainage. Paper presented at the 7th International Conference on Acid Rock Drainage (ICARD), St. Louis, MO http://www.asmr.us/Publications/Conference%20Proceedings/2006/2618-Zinck-ON-2.pdf [Google Scholar]
- U.S. EPA. (2012d). Cement kiln dust waste. Wastes—non-hazardous waste—industrial waste. Retrieved September 2014, from http://www.epa.gov/osw/nonhaz/industrial/special/ckd/index.htm [Google Scholar]
- U.S. EPA. (2012e). Aluminum production wastes. Radiation protection. Retrieved September 2014, from http://www.epa.gov/radiation/tenorm/aluminum.html [Google Scholar]
- Sutar H, Mishra SH, Sahoo SK, Chakraverty AP, and Maharana HS (2014). Progress of red mud utilization: An overview. Am. Chem. Sci. J, 4, 25–279. [Google Scholar]
- Gray CW, Dunham SJ, Dennis PG, Zhao FJ, and McGrath S (2006). Field evaluation of in situ remediation of a heavy metal contaminated soil using lime and red-mud. Environ. Poll, 142, 530–539. [DOI] [PubMed] [Google Scholar]
- Muller I, and Pluquet E (1998). Water. Sci. China Ser. D, 37, 379–386. [Google Scholar]
- Lombi E, Zhao FJ, Zhang G, Sun B, Fitz WJ, Zhang H, and McGrath SP (2002). In situ fixation of metals in soils using bauxite residue: Chemical assessment. Environ. Poll, 118, 435–443. [DOI] [PubMed] [Google Scholar]
- Friesl W, Lombi E, Horak O, and Wenzel WW (2003). Immobilization of heavy metals in soils using inorganic amendments in a greenhouse study. J. Plant Nutr. Soil Sci, 166, 191–196. [Google Scholar]
- Basta NT, and McGowen SL (2004). Evaluation of chemical immobilization treatments for reducing heavy metal transport in a smelter-contaminated soil. Environ. Poll, 127, 73–82. [DOI] [PubMed] [Google Scholar]
- Dermatas D, and Meng XG (1996). Stabilization/Solidification (S/S) of heavy metal contaminated soils by means of a quicklime-based treatment approach. Stabilization and solidification of hazardous, radioactive, and mixed wastes, ASTM STP 1240, 1. Paper presented at the American Society for Testing and Materials, Philadelphia. [Google Scholar]
- Li YM, Chaney RL, Siebielec G, and Kerschner BA (2000). Response of four turfgrass cultivars to limestone and biosolids-compost amendment of a zinc and cadmium contaminated soil at Palmerton, Pennsylvania. J. Environ. Qual, 29, 1440–1447. [Google Scholar]
- Pierzinsky GM, and Schwab AP (1993). Bioavailability of zinc cadmium and lead in a metal-contaminated alluvial soil. J. Environ. Qual, 22, 247–254. [Google Scholar]
- Sanchez AG, and Ayuso EA (2008). Soil remediation in mining polluted areas. Conferencia invitada revista de la sociedad espanola de mineralogia, 10, 76–84. [Google Scholar]
- Trakal L, Neuberg M, Tlustoš P, Száková J, Tejnecký V, and Drábek O (2011). Dolomite limestone application as a chemical immobilization of metal-contaminated soil. Plant Soil Environ., 57, 173–179. [Google Scholar]
- Girovish MJ (1996). Biosolids treatment and management: Processes for beneficial use. New York: Marcel Dekker, Inc. [Google Scholar]
- Basta NT, Gradwohl R, Snethen KL, and Schroder JL (2001). Chemical immobilization of lead, zinc, and cadmium in smelter-contaminated soils using biosolids and rock phosphate. J. Environ. Qual, 30, 1222–1230. [DOI] [PubMed] [Google Scholar]
- National Lime Association. (2014). Biosolids and sludge. Retrieved August, from http://www.lime.org/uses_of_lime/environmental/biosolids.asp [Google Scholar]
- Lal R (Ed.). (2006). Encyclopedia of soil science (2nd ed.). New York, NY: Taylor and Francis Group, LLC. [Google Scholar]
- National Slag Association. (2013). Common uses for slag. Retrieved September 2014, from http://www.nationalslag.org/common-uses-slag [Google Scholar]
- Penn State, College of Agricultural Sciences. (2014). Penn State Extension. Slag. Nontraditional soil amendments. Retrieved 23 July 2014, from http://extension.psu.edu/agronomy-guide/cm/sec9/sec96 [Google Scholar]
- Iskander IK (2001). Environmental restoration of metals-contaminated soils. Boca Raton, FL: CRC Press LLC, Lewis Publishers. [Google Scholar]
- Sappin-Didier V (1995). Utililisation de composes inorganiques pour diminuer les flux de metaux dan deux agrosystems pollues: Etude des mecanismes impliques par l'emploi d'un compose du fer, PhD Thesis. As cited in Iskander IK (2001), Environmental restoration of metals-contaminated soils. Lewis Publishers. [Google Scholar]
- Mench MJ, Didier VL, Loffler M, Gomez A, and Masson P (1994). A mimicked in-situ remediation study of metal-contaminated soils with emphasis on cadmium and lead. J. Environ. Qual, 23, 58–63. [Google Scholar]
- Mench M, Vangronsveld J, Clijster H, Lepp NW, and Edwards R (1999). In-situ metal immobilization and phytostabilisation of contaminated soils In Logan T, Banuelos G, Vangronsveld J, and Terry N (Eds.), Phytoremediation of contaminated soils and water. Boca Raton, FL: CRC Press. [Google Scholar]
- Muller I, and Pluquet E (1997). Immobilization of heavy metals in mud dredged from a seaport. Rotterdam: Int. Conf. Contaminated Sediments, September/7-11/1997. [Google Scholar]
- Mench M, Vangronsveld J, Lepp NW, and Edwards R (1998). Physico-chemical aspects and efficiency of trace element immobilization by soil amendments In Vangronsveld J and Cunningham SD (Eds.), Metal contaminated soils: In situ inactivation and phytorestoration (pp. 151–182). New York: Spring. [Google Scholar]
- De Boodt MF (1991). Application of the sorption theory to eliminate heavy metals from waste waters and contaminated soils, Interactions at the soil colloid-soil solution interface In G. H. Bolt, De Boodt MF, Hayes MHB, and McBride MB (Eds.), NATO ASI series, series E: Applied sciences (Vol. 190, pp. 293–320). Dordrecht (NL): Luwer Academic Publishers. [Google Scholar]
- Kumar A (2004). Environmental contamination and bioreclamation. New Delhi, India: APH Publishing Corporation. [Google Scholar]
- Stegmann R, Brunner G, Calmano W, and Matz G (2001). Treatment of contaminated soil: Fundamentals, analysis, applications. New York, NY: Springer-Verlag Berlin Heidelberg. [Google Scholar]
- U.S. EPA. (2013c). Foundry sand. Wastes—resource conservation—industrial materials recycling. Retrieved September 2014, from http://www.epa.gov/epawaste/conserve/imr/foundry/ [Google Scholar]
- Merck. (1983). The Merck index (10th ed.). Rahway, NJ: Merck and Co. [Google Scholar]
- Stillwell DE, and Ranciato JF (2008). Use of phosphates to immobilize lead in community garden soils. The Connecticut Agricultural Experiment Station, New Haven: Bulletin 1018. Department of Analytical Chemistry. [Google Scholar]
- Hettiarachchi GM, and Pierzynski GM (2004). Soil lead bioavailability and in situ remediation of lead-contaminated soils: A review. Environ. Prog, 23, 78–93. [Google Scholar]
- Ma QY, Traina SJ, Logan TJ, and Ryan JA (1993). In-Situ lead immobilization by apatite. Environ. Sci. Technol, 27, 1803–1810. [Google Scholar]
- Chlopecka A, and Adriano DC (1996). Mimicked in-situ stabilization of metals in a cropped soil: Bioavailability and chemical form of zinc. Environ. Sci. Technol, 30, 3294–3303. [Google Scholar]
- Mench MJ, Manceau A, Vangronsveld J, Clijsters H, and Mocquot B (2000). Capacity of soil amendments in lowering the phytoavailability of sludge-born zinc. Agronomie, 20, 383–397. [Google Scholar]
- Naidu R, Bolan N, Kookana RS, and Tiller KG (1994). Ionic strength and pH effects on the adsorption of cadmium and the surface charge of soils. Eur. J. Soil Sci, 45, 419–429. [Google Scholar]
- Naidu R, Lamb DT, Bolan NS, and Gawandar J (2012). Recovery and reuse of phosphorous from wastewater. Retrieved from http://www.massey.ac.nz/~flrc/workshops/12/Manuscripts/Naidu_2012.pdf
- Abollino O, Giacomino A, Malandrina M, and Mentaste E (2007). The efficiency of verminculite as natural sorbent for heavy metals. Application to a contaminated soil. Water Air Soil Poll., 181, 149–160. [Google Scholar]
- Ciccu R, Ghiani M, Serci A, Fadda S, Peretti R, and Zucca A (2003). Heavy metal immobilization in the mining-contaminated soils using various industrial wastes. Miner. Eng, 16, 187–192. [Google Scholar]
- Gray CW, Dunham SJ, Dennis PG, Zhao FJ, and McGrath S (2006). Field evaluation of in situ remediation of a heavy metal contaminated soil using lime and red-mud. Environ. Poll, 142, 530–539. [DOI] [PubMed] [Google Scholar]
- Illera V, Garrido F, Serrano S, and Garcia-Gonzalez MT (2004). Immobilization of the heavy metals Cd, Cu and Pb in an acid soil amended with gypsum- and lime-rich industrial by-products. Eur. J. Soil Sci, 55, 135–145. [Google Scholar]
- Lombi E, Hamon RE, McGrath SP, and McLaughlin MJ (2003). Lability of Cd, Cu, and Zn in polluted soils treated with lime, beringite, and red mud and identification of a non-labile colloidal fraction of metals using isotopic techniques. Environ. Sci. Technol, 37, 979–984. doi: 10.1021/Es026083w [DOI] [PubMed] [Google Scholar]
- Florida Industrial and Phosphate Research Institute (FIPR). (2010). Phosphate primer. Retrieved 12 September 2014, from http://www1.fipr.state.fl.us/PhosphatePrimer/0/684AE64864D115FE85256F88007AC781 [Google Scholar]
- Elliott HA, and Dempsey BA (1991). Agronomic effects of land application of water treatment sludges. J. Am. Water Works Assoc, 84, 126–131. [Google Scholar]
- Chiang YW, Ghyselbrecht K, Santos RM, Martens JA, Swennen R, Cappuyns V, and Meesschaert B (2012). Adsorption of multi-heavy metals onto water treatment residuals: Sorption capacities and applications. Chem. Eng. J, 200–202, 405–415. [Google Scholar]
- Fan JH, He ZL, Ma LQ, Yang YG, Yang XE, and Stoffella PJ (2011). Immobilization of copper in contaminated sandy soils using calcium water treatment residue. J. Hazard Mater, 189, 710–718. [DOI] [PubMed] [Google Scholar]
- Rengasamy P, Oades JM, and Hancock TW (1980). Improvement of Soil Structure and Plant-Growth by Addition of Alum Sludge. Commun. Soil Sci. Plan, 11, 533–545. [Google Scholar]
- Silveira ML, Miyittah MK, and O'Connor SGA (2006). Phosphorus release from a manure-impacted spodosol: Effect of a water treatment residual. J. Environ. Qual, 35, 529–541. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhao Y, and Pei Y (2012). Investigation on reusing water treatment residuals to remedy soil contaminated with multiple metals in Baiyin, China. J. Hazard Mater, 237–238, 240–246. [DOI] [PubMed] [Google Scholar]
- Lombi E, Hamon RE, Wieshammer G, McLaughlin M, and McGrath S (2004). Assessment of the use of industrial by-products to remediate a copper- and arsenic-contaminated soil. J. Environ. Qual, 33, 902–910. [DOI] [PubMed] [Google Scholar]
- Bünemann EK, Schwenke GD, and Van Zwietan L (2006). Impact of agricultural inputs on soil organisms: A review. Aust. J. Soil Res, 44, 379–406. [Google Scholar]
- Sud D, Mahajan G, and Kaur MP (2008). Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—A review. Bioresource Technol., 99, 6017–6027. [DOI] [PubMed] [Google Scholar]
- Novak J, Ro K, Ok YS, Sigua G, Spokas K, Uchimiya S, and Bolan N (2016). Biochars multifunctional role as a novel technology in the agricultural, environmental, and industrial sectors. Chemosphere, 142, 1–3. [DOI] [PubMed] [Google Scholar]
- Bishnoi NR, Bajaj M, Sharma N, and Gupta A (2004). Adsorption of chromium (VI) on activated rice husk carbon and activated alumina. Bioresour. Technol, 91, 305–307. [DOI] [PubMed] [Google Scholar]
- Zheng RL, Chen Z, Cai C, Wang XH, Huang YZ, Xiao B, and Sun GX (2013). Effect of biochars from rice husk, bran, and straw on heavy metal uptake by pot-grown wheat seedling in a historically contaminated soil. Bioresources, 8, 5965–5982. [Google Scholar]
- Montanher SF, Oliveira EA, and Rollemberg MC (2005). Removal of metal ions from aqueous solutions by sorption onto rice bran. J. Hazard Mater, 117, 207–211. [DOI] [PubMed] [Google Scholar]
- Oliveira EA, Montanher SF, Andrade AD, Nobrega JA, and Rollemberg MC (2005). Equilibrium studies for the sorption of chromium and nickel from aqueous solutions using raw rice bran. Process Biochem., 40, 3485–3490. [Google Scholar]
- Roy D, Greenlaw PN, and Shane BS (1993). Adsorption of heavy-metals by green-algae and ground rice hulls. J. Environ. Sci. Heal. A, 8, 37–50. [Google Scholar]
- Basci N, Kocadagistan E, and Kocadagistan B (2003). Biosorption of Cu II from aqueous solutions by wheat shells. Desalination, 164, 135–140. [Google Scholar]
- Farajzadeh MA, and Monji AB (2004). Adsorption characteristics of wheat bran towards heavy metal cations. Sep. Purif. Technol, 38, 197–207. [Google Scholar]
- Gardea-Torresdey JL, Tiemann KJ, Armendariz V, Bess-Oberto L, Chianelli RR, Rios J, Gamez G (2000). Characterization of chromium (VI) binding and reduction to chromium(III) by the agricultural byproduct of Avena monida (oat) biomass. J. Hazard. Mater, 80, 175–188. [DOI] [PubMed] [Google Scholar]
- Garg VK, Gupta R, Kumar R, and Gupta RK (2004). Adsorption of chromium from aqueous solution on treated sawdust. Bioresour. Technol, 92, 79–81. [DOI] [PubMed] [Google Scholar]
- Manju GN, and Anirudhan TS (1997). Use of coconut fiber pith-based pseudo activated carbon for chromium (VI) removal. Ind. J. Environ. Health, 4, 289–298. [Google Scholar]
- Tsui MT, Cheung KC, Tam NF, and Wong MH (2006). A comparative study on metal sorption by brown seaweed. Chemosphere, 65, 51–57. [DOI] [PubMed] [Google Scholar]
- Hashem A, Abdel-Halim ES, El-Tahlawy KF, and Hebeish A (2005). Enhancement of adsorption of Co (II) and Ni (II) ions onto peanut hulls though esterification using citric acid. Adsorp. Sci. Technol, 23, 367–380. [Google Scholar]
- Wilson K, Yang H, Seo CW, and Marshall WE (2006). Select metal adsorption by activated carbon made from peanut shells. Bioresour. Technol, 97, 2266–2270. [DOI] [PubMed] [Google Scholar]
- Cimino G, Passerini A, and Toscano G (2000). Removal of toxic cations and Cr(Vi) from aqueous solution by hazelnut shell. Water Res., 34, 2955–2962. [Google Scholar]
- Demirbas E, Kobya M, Oncel S, and Sencan S (2002). Removal of Ni(II) from aqueous solution by adsorption onto hazelnut shell activated carbon: Equilibrium studies. Bioresour. Technol, 84, 291–293. [DOI] [PubMed] [Google Scholar]
- Johns MM, Marshall WE, and Toles CA (1998). Agricultural byproducts as granular activated carbons for adsorbing dissolved metals and organics. J. Chem. Technol. Biotechnol, 71, 131–140. [Google Scholar]
- Hashem A, Aly AA, Aly AS, and Hebeish A (2006). Quaternization of cotton stalks and palm tree particles for removal of acid dye from aqueous solutions. Polym-Plast Technol., 45, 389–394. [Google Scholar]
- Goswami S, and Ghosh UC (2005). Studies on adsorption behaviour of Cr(VI) onto synthetic hydrous stannic oxide. Water SA, 31, 597–602. [Google Scholar]
- Ribas LCC, Mendonca MM, Camelini CM, and Soares CHL (2009). Use of spent mushroom substrates from Agaricus subrufescens (syn. A. blazei, A. brasiliensis) and Lentinula edodes productions in the enrichment of a soil-based potting media for lettuce (Lactuca sativa) cultivation: Growth promotion and soil bioremediation. Bioresour. Technol, 100, 4750–4757. [DOI] [PubMed] [Google Scholar]
- Beesley L, Inneh O, Norton G, Moreno-Jimenez E, Pardo T, Clemente R, and Dawson J (2014). Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ. Pollut, 186, 195–202. [DOI] [PubMed] [Google Scholar]
- Burgos P, Madejon P, Cabrera F, and Madejon E (2010). By-products as amendment to improve biochemical properties of trace element contaminated soils: Effects in time. Int. Biodeter. Biodegr, 64, 481–488. [Google Scholar]
- Akkajit P, DeSutter T, and Tongcumpou C (2013). Short-term effects of sugarcane waste products from ethanol production plant as soil amendments on sugarcane growth and metal stabilization. Environ. Sci. Process Impacts, 15, 947–954. [DOI] [PubMed] [Google Scholar]
- Akkajit P, DeSutter T, and Tongcumpou C (2014). Effects of sugarcane waste-products on Cd and Zn fractionation and their uptake by sugarcane (Saccharum officinarum L.). Environ. Sci. Process. Impacts, 1, 88–93. [DOI] [PubMed] [Google Scholar]
- Niu X, Zheng L, Zhou J, Dang Z, and Li Z (In Press). Synthesis of an adsorbent from sugarcane bagass by graft copolymerization and its utilization to remove Cd(II) ions from aqueous solution. J. Taiwan Inst. Chem. E, 46, 156–164. [Google Scholar]
- Mata YN, Blazquez ML, Ballester A, Gonzalez F, and Munoz JA (2009). Sugar-beet pulp pectin gels as biosorbent for heavy metals: Preparation and determination of biosorption and desorption characteristics. Chem. Eng. J, 150, 289–301. [Google Scholar]
- Gurgel LVA, de Freitas RP, and Gil LF (2008). Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by sugarcane bagasse and mercerized sugarcane bagasse chemically modified with succinic anhydride. Carbohyd. Polym, 74, 922–929. [Google Scholar]
- Garrido F, Illera V, Campbell CG, and Garcia-Gonzalez MT (2006). Regulating the mobility of Cd, Cu and Pb in an acid soil with amendments of phosphogypsum, sugar foam, and phosphoric rock. Eur. J. Soil Sci, 57, 95–105. [Google Scholar]
- Jimenez Moraza C, Iglesias N, and Palencia I (2006). Application of sugar foam to a pyrite-contaminated soil. Miner. Eng, 19, 399–406. [Google Scholar]
- Júnior O, Gurgel L, Freitas R, and Gil L (2006). Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by mercerized cellulose and mercerized sugarcane bagasse chemically modified with EDTS dianhydride (EDTAD). Carbohydr. Polym, 77, 643–650. [Google Scholar]
- Gupta VK, and Ali I (2000). Utilisation of bagasse fly ash (a sugar industry waste) for the removal of copper and zinc from wastewater. Sep. Purif. Technol, 18, 131–140. [Google Scholar]
- Maranon E, and Sastre H (1991). Heavy-Metal Removal in Packed-Beds Using Apple Wastes. Bioresource Technol., 38, 39–43. [Google Scholar]
- Annadurai G, Juang RS, and Lee DL (2002). Adsorption of heavy metals from water using banana and orange peels. Water Sci. Technol, 47, 185–190. [PubMed] [Google Scholar]
- Macchi G, Marani D, and Tiravanti G (1986). Uptake of mercury by exhausted coffee grounds. Environ. Technol. Lett, 7, 431–444. [Google Scholar]
- Erwan Ismail, Saud MR, H. M., Othman R, Habib SH, Kausar Hand Naher L (2013). Effect of oil palm frond compost amended coconut coir dust soilless growing media on growth and yield of cauliflower. Int. J. Agric. Biol, 15, 731–736. [Google Scholar]
- USBI. (2014). Manufactures and retailers. Retrieved August 2014, from http://biochar-us.org/manufacturers-retailers [Google Scholar]
- USA Rice Federation. (2014). The U.S. rice industry at-a-glance. Retrieved August, 2014, from http://www.usarice.com/doclib/188/219/3674.pdf [Google Scholar]
- American Olive Oil Producers Association (AOOPA). (2014). The American olive oil industry facts. Retrieved August 21, 2014, from http://www.aoopa.org/U.S.OliveOilProductionFacts-i-17-2.html [Google Scholar]
- Alburquerque JA, de la Fuente C, and Bernal MP (2011). Improvement of soil quality after “alperujo” compost application to two contaminated soils characterised by differing heavy metal solubility. J. Environ. Manage, 92, 733–741. [DOI] [PubMed] [Google Scholar]
- Media Manta. (2014). Silk (raw) production and silkworm farms in the United States. Featured company listings. Retrieved September 5, 2014, from http://www.manta.com/mb_35_C011702U_000/silk_raw_production_and_silkworm_farm [Google Scholar]
- U.S. Department of Agriculture, Economic Research Service. (2012). Sugar and sweeteners. Retrieved September 2014, from http://www.ers.usda.gov/topics/crops/sugar-sweeteners/background.aspx#.VAdNjvldUy4 [Google Scholar]
- Hodson ME, Valsami-Jones É, and Cotter-Howells JD (2000). Bonemeal additions as a remediation treatment for metal contaminated soil. Environ. Sci. Technol, 34, 3501–3507. [Google Scholar]
- U.S. EPA. (2012c). Beneficial use of waste materials: State of the practice 2012. U.S. Environmental Protection Agency (U.S. EPA). [Google Scholar]
- Aguilar-Carrillo J, Barrios L, Garrido F, and Garcia-Gonzalez MT (2007). Effects of industrial by-product amendments on As, Cd and Tl retention/release in an element-spiked acidic soil. Appl. Geochem, 22, 1515–1529. [Google Scholar]
- Bert V, Lors C, Ponge JF, Caron L, Biaz A, Dazy M, and Masfaraud JF (2012). Metal immobilization and soil amendment efficiency at a contaminated sediment landfill site: A field study focusing on plants, springtails, and bacteria. Environ. Poll, 169, 1–11. [DOI] [PubMed] [Google Scholar]
- Bian R, Joseph S, Cui L, Pan G, Li L, Liu X, and Donne S (2014). A three-year experiment confirms continuous immobilization of cadmium and lead in contaminated paddy field with biochar amendment. J. Hazard Mater, 272, 121–128. [DOI] [PubMed] [Google Scholar]
- Bian R, Chen D, Liu X, Cui L, Li L, Pan G, and Chang A (2013). Biochar soil amendment as a solution to prevent Cd-tainted rice from China: Results from a cross-site field experiment. Ecol. Eng, 58, 378–383. [Google Scholar]
- Bradham KD, Laird B, Rasmussen PE, Schoof RA, Serda SM, Siciliano SD, and Hughes MF (2013). Workshop Report: Assessing the bioavailability and risk from metal contaminated soils and dusts. Research Triangle Park, NC: U.S. EPA. [Google Scholar]
- Chaney RL, Brown S, Mahoney M, Compton H, and Sprenger M (2012). Using organic amendments, byproducts and agronomy in remediation of hardrock mining sites. Paper presented at the EPA Hardrock Mining Conference. [Google Scholar]
- Garau G, Silvetti M, Castaldi P, Mele E, Deiana P, and Deiana S (2014). Stabilising metal(loid)s in soil with iron and aluminium-based products: Microbial, biochemical and plant growth impact. J. Environ. Manage, 139, 146–153. [DOI] [PubMed] [Google Scholar]
- Geebelen W, Sappin-Didier V, Ruttens A, Carleer R, Yperman J, Bongue-Boma K, Mench M, van der Lelie N, and Vangronsveld J (2006). Evaluation of cyclonic ash, commercial Na-silicates, lime and phosphoric acid for metal immobilisation purposes in contaminated soils in Flanders (Belguim). Environ. Poll, 144, 32–39. [DOI] [PubMed] [Google Scholar]
- Gonzalez V, Garcia I, Del Moral F, and Simon M (2012). Effectiveness of amendments on the spread and phytotoxicity of contaminants in metal–arsenic polluted soil. J. Hazard Mater, 205–206, 72–80. [DOI] [PubMed] [Google Scholar]
- Gu HH, Qiu H, Tian T, Zhan SS, Deng THB, Chaney RL, and Qiu RL (2011). Mitigation effects of silicon rich amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on multi-metal contaminated acidic soil. Chemosphere, 83, 1234–1240. [DOI] [PubMed] [Google Scholar]
- Houben D, Pircar J, and Sonnet P (2012). Heavy metal immobilization by cost-effective amendments in a contaminated soil: Effects on metal leaching and phytoavailability. J. Geochem. Explor, 123, 87–94. [Google Scholar]
- Hwang T, and Neculita CM (2013). In situ immobilization of heavy metals in severely weathered tailings amended with food waste-based compost and zeolite. Water, Air, Soil Poll., 224, 1388. [Google Scholar]
- Lee SH, Kim EY, Park H, Yun J, and Kim JG (2011). In situ stabilization of arsenic and metal-contaminated agricultural soil using industrial by-products. Geoderma, 161, 1–7. [Google Scholar]
- Lee SH, Park H, Koo N, Hyun S, and Hwang A (2011b). Evaluation of the effectiveness of various amendments on trace metals stabilization by chemical and biological methods. J. Hazard Mater, 188, 44–51. [DOI] [PubMed] [Google Scholar]
- Lee SH, Li W, Lee WS, Koo N, Koh IH, Kim MS, and Park JS (2014). Influence of amendments and aided phytostabilization on metal availability and mobility in Pb/Zn mine tailings. J. Environ. Manage, 139, 15–21. [DOI] [PubMed] [Google Scholar]
- Liu R, and Lal R (2012). Nanoenhanced Materials for Reclamation of Mine Lands and Other Degraded Soils: A Review. J. Nanotechnol, 2012. [Google Scholar]
- Makela M, Harju-Oksanen ML, Watkins G, Ekroos A, and Dahl O (2012). Feasibility assessment of inter-industry solid residue utilization for soil amendment-Trace element availability and legislative issues. [Article]. Resour. Conserv. Recy, 67, 1–8. [Google Scholar]
- Mallampati SR, Mitoma Y, Okuda T, Sakita S, and Kakeda M (2013). Total immobilization of soil heavy metals with nano-Fe/Ca/CaO dispersion mixtures. Environ. Chem. Lett, 11, 119–125. [Google Scholar]
- Osama N, Michel M, Clemence B, Mikael MH, Fouad A, Frederic H, and Philippe LC (2012). In situ stabilization of trace metals in a copper-contaminated soil using P-spiked Linz–Donawitz slag. Environ. Sci. Poll. Res, 19, 847–857. [DOI] [PubMed] [Google Scholar]
- Oste L, Lexmond TM, and Van Riemsdijk WH (2002). Metal immobilization in soils using synthetic zeolites. J. Environ. Qual, 31, 813–821. [DOI] [PubMed] [Google Scholar]
- Pardo T, Bernal MP, and Clemente R (2014). Efficiency of soil organic and inorganic amendments on the remediation of a contaminated mine soil: I. Effects on trace elements and nutrients solubility and leaching risk. Chemosphere, 107, 121–128. [DOI] [PubMed] [Google Scholar]
- Soler-Rovira P, Madejon E, Madejon P, and Plaza C (2010). In situ remediation of metal-contaminated soils with organic amendments: Role of humic acids in copper bioavailability. Chemosphere, 79, 844–849. [DOI] [PubMed] [Google Scholar]
- Sun Y, Sun G, Xu Y, Wang L, Liang X, and Lin D (2013). Assessment of sepiolite for immobilization of cadmium-contaminated soils. Geoderma, 193–194, 149–155. [Google Scholar]
- Wang F, Ouyang W, Hao F, Lin C, and Song N (2014). In situ remediation of cadmium-polluted soil reusing four by-products individually and in combination. J. Soils Sediments, 14, 451–461. [Google Scholar]
- Zhang M, and Pu J (2011). Mineral materials as feasible amendments to stabilize heavy metals in polluted urban soils. J. Environ. Sci, 23, 607–615. [DOI] [PubMed] [Google Scholar]
- Zhou H, Zhou X, Zeng M, Liao BH, Liu L, Yang WT, and Wang YJ (2014). Effects of combined amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on contaminated paddy soil. Ecotox. Environ. Safe, 101, 226–232. [DOI] [PubMed] [Google Scholar]
- Hamon R, McLaughlin M, and Lombi E (2007). Natural attenuation of trace element availability in soils. Society of Environmental Toxicology and Chemistry (SETAC) Books. [Google Scholar]
- Kabata-Pendias A (2011). Trace elements in soils and plants (4th ed.). Boca Raton, FL: Taylor and Francis Group, LLC. [Google Scholar]
- Langmuir DL, Chrostrowski P, Vigneault B, and Chaney RL (2005). Issue paper on environmental chemistry of metals: U.S. EPA risk assessment forum. Papers addressing scientific issues in the risk assessment of metals. [Google Scholar]
- Essington ME, and Mattigod SV (1991). Trace-element solid-phase associations in sewage-sludge and sludge-amended soil. Soil Sci. Soc. Am. J, 55, 350–356. [Google Scholar]
- Hettiarachchi GM, Ryan JA, Chaney RL, and La Fleur CM (2003). Sorption and desorption of cadmium by different fractions of biosolids-amended soils. J. Environ. Qual, 32, 1684–1693. [DOI] [PubMed] [Google Scholar]
- Lombi E, Zhao FJ, Wieshammer G, Zhang GY, and McGrath SP (2002b). In situ fixation of metals in soils using bauxite residue: Biological effects. Environ. Poll, 118, 445–452. [DOI] [PubMed] [Google Scholar]
- McKenzie RM (1980). The adsorption of lead and other heavy metals on oxides of manganese and iron. Aust. J. Soil Res, 18, 61–73. [Google Scholar]
- Lindsay WL (2001). Chemical equilibria in soils. Caldwell, NJ: The Blackburn Press. [Google Scholar]
- Houben D, Pircar J, and Sonnet P (2012). Heavy metal immobilization by cost-effective amendments in a contaminated soil: Effects on metal leaching and phytoavailability. J. Geochem. Explor, 123, 87–94. [Google Scholar]
- U.S. EPA. (2008). Beneficial reuse of industrial byproducts in the gulf coast region. Fairfax, VA: ICF International. [Google Scholar]
- Chaney RL, Reeves PG, Ryan JA, Simmons RW, Welch RM, and Angle JS (2004). An improved understanding of soil Cd risk to humans and low cost methods to remediate soil Cd risks. BioMetals, 17, 549–553. [DOI] [PubMed] [Google Scholar]
- McKenna IM, Chaney RL, Tao SH, Leach RM Jr., and Williams FM (1992). Interactions of plant zinc and plant species on the bioavailability of plant cadmium to Japanese quail fed lettuce and spinach. Environ. Res, 57, 73–87. [DOI] [PubMed] [Google Scholar]
- Stuczynski TI, Siebielec G, Daniels WL, McCarty GC, and Chaney RL (2007). Biological aspects of metal waste reclamation with sewage sludge. J. Environ. Qual, 36, 1154–1162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.