Extended Data Fig. 6. AKAP95 condensation requires Tyrosine in 101–210 and regulates splicing.
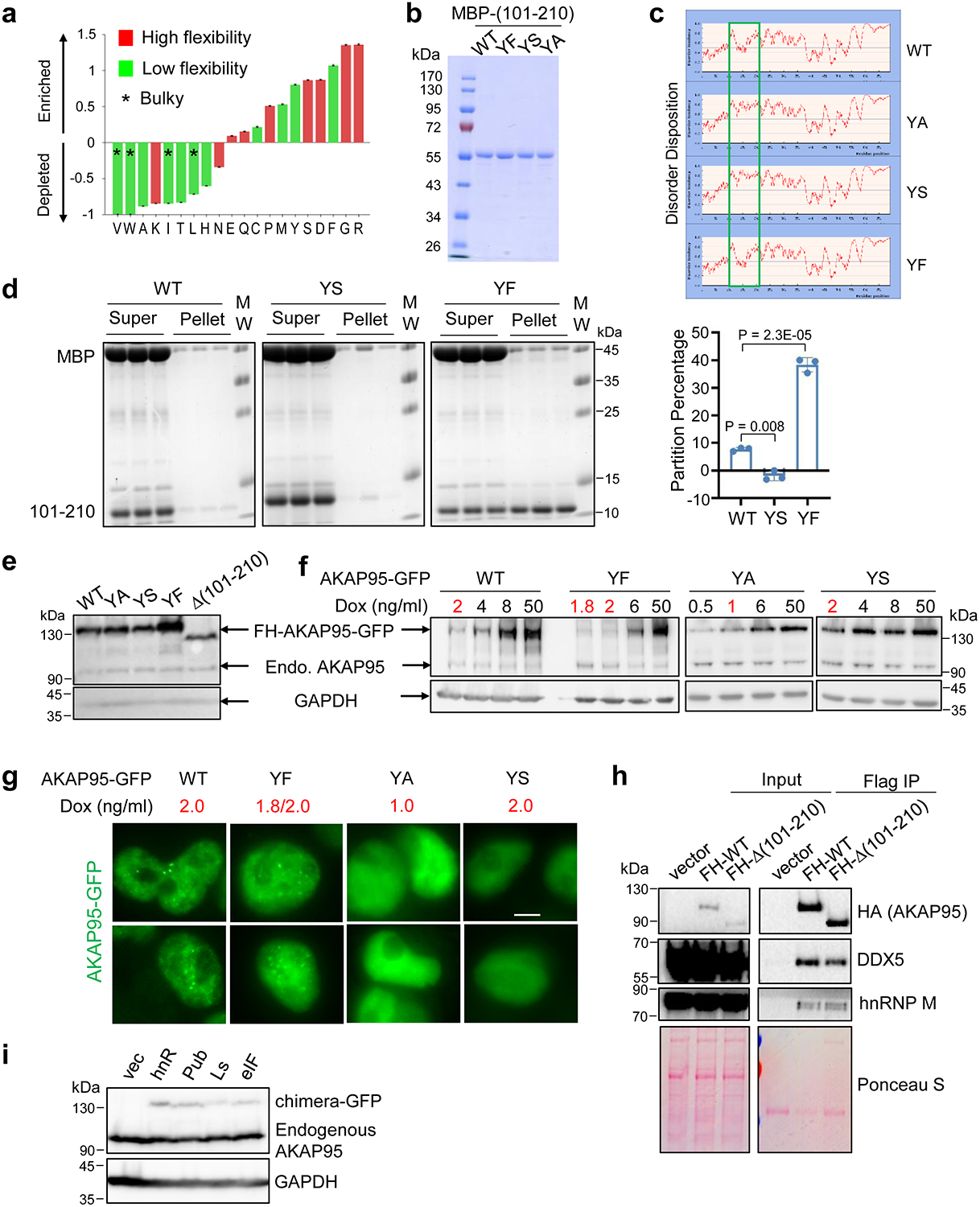
a, Amino acid enrichment for AKAP95 (101–210). By Composition Profiler, using SwissProt 51 Dataset as background. b, Purified MBP fused to AKAP95 (101–210) WT and mutants were resolved on SDS-PAGE and stained with Coomassie blue. Repeated 2 times. c, Disorder plot of AKAP95 WT or mutants with indicated mutations in 101–210. d, MBP-AKAP95 (101–210) WT, YS, and YF, all at 35 μM and in 30 mM NaCl, were treated with TEV protease for 2 hrs in 3 independent assays, and subjected to centrifugation. The supernatant and pellets (resuspended in the same volume as the supernatant) were resolved by SDS-PAGE followed with coomassie blue staining. MBP signal in the pellet reflects residual supernatant fraction, and its percentage [MBP pellet/(supernatant + pellet)] was subtracted from the (101–210) pellet percentage. Such normalized (101–210) pellet percentages are plotted as Partition Percentage as mean ± SD of n = 3 independent experiments. It is most likely that all supernatants may also have substantial portion of condensates. Moreover, the size cutoff of condensates is also arbitrary, as protein assemblies may take a continuum of size distribution71. e, Immunoblotting by α-AKAP95 (top) or GAPDH (bottom) of total lysates from Flp-In T-Rex 293 cell lines induced to express full-length AKAP95 WT or indicated mutants fused to GFP. Repeated 3 times. f,g, Indicated full-length AKAP95 WT or mutants fused to GFP were induced by various concentrations of doxycycline in Flp-In T-Rex 293 cell lines. Immunoblotting of total cell lysates with indicated antibodies (f). The doxycycline concentrations in red font activated the transgene at the near endogenous level, and were selected for treating cells and fluorescence microscopy assays of fixed cells in (g). Scale bar, 5 μm. Repeated 2 times. h, 293T cells transiently expressing indicated constructs with FLAG-HA-tag were used for α-FLAG immunoprecipitation and immunoblotting with indicated antibodies and Ponceau S staining. Repeated 2 times. i, Immunoblotting of 293T cells transfected with empty vector or indicated FLAG-HA-tagged AKAP95 chimeras fused to GFP. Bottom, by anti-GAPDH. Top, by anti-AKAP95 (Bethyl Laboratories, A301–062A, recognizes an epitope in a region between residue 575 and 625 of human AKAP95). Repeated 2 times. P values by two-sided Student’s t-test for d. Uncropped blots and statistical source data are provided as in source data extended data fig 6.
